
ŹµŃéŹŅæÉŅŌĶعżČżĢõĶ¾¾¶Ą“ÖĘČ”N2£ŗ
¢Ł¼ÓČČĢõ¼žĻĀÓĆNH3»¹ŌCuOÖĘµĆ“æ¾»µÄN2ŗĶĶ·Ū£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
![]()
¢Ś ½«æÕĘųĶعż×ĘČȵÄĶ·ŪÖʵƵÄN2
¢Ū ¼ÓČČNaNO2£ØÓŠ¶¾ŠŌ£©ÓėNH4ClµÄ»ģŗĻÅØČÜŅŗÖĘČ”N2£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
![]()
°“ÉĻŹöĶ¾¾¶ÖĘN2æɹ©Ń”ŌńµÄŹµŃéŅĒĘ÷ČēĻĀĶ¼ĖłŹ¾£¬ĘäĖū±ŲŅŖµÄŅĒĘ÷ČēĢś¼ÜĢØ”¢Ģś¼Š”¢ĢśČ¦”¢ŹÆĆŽĶų”¢¾Ę¾«µĘµČĪ“ĮŠ³ö”£
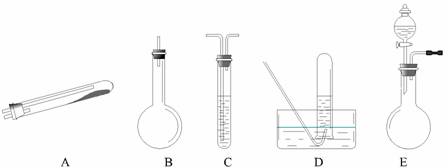
£Ø1£©°“Ķ¾¾¶ ¢Ł ÖĘČ”N2Ź±ĖłŠčµÄ°±ĘųŅŖÓĆÅØ°±Ė®ÓėÉśŹÆ»Ņ·“Ó¦µĆµ½£¬»Æѧ·½³ĢŹ½ĪŖ£ŗ
CaO+NH3?H2O === Ca(OH)2+NH3”ü£¬£¬×īŗĆŅŖÓĆÉĻŹö×°ÖĆÖŠµÄ ”£ £ØĢī×ÖÄø£¬ĻĀĶ¬£©×÷ĪŖ°±Ęų·¢Éś×°ÖĆ”£ŅŖÖĘČ”²¢ŹÕ¼ÆN2£¬»¹Ó¦Ź¹ÓƵ½ÉĻŹö×°ÖĆÖŠµÄ
£Ø2£©¢ŁŗĶ¢ŚĮ½ÖÖĶ¾¾¶³£³£±»ĮŖŗĻŹ¹ÓĆ”£ÕāÖÖ·½·ØÓėĶ¾¾¶¢ŪĻą±ČĘäÓÅŌ½ŠŌŌŚÓŚ ”£
£Ø3£©¼ģ²éE×°ÖĆĘųĆÜŠŌµÄ·½·ØŹĒ ”£ĄūÓĆE×°ÖĆ»¹æÉŅŌÖĘČ”µÄĘųĢåÓŠ £ØÖ»ŠčŠ“³öČżÖÖ£©”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅæÉŅŌĶعżČżĢõĶ¾¾¶Ą“ÖĘČ”µŖĘų£ŗ
¢Ł¼ÓČČĢõ¼žĻĀÓĆNH3»¹ŌCuOÖĘµĆ“æ¾»µÄN2ŗĶ»īŠŌĶ·Ū£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
![]()
¢Ś ½«æÕĘųĶعż×ĘČȵĻīŠŌĶ·ŪÖĘµĆ½Ļ“æ¾»µÄN2
¢Ū ¼ÓČČNaNO2£ØÓŠ¶¾ŠŌ£©ÓėNH4ClµÄ»ģŗĻÅØČÜŅŗÖĘČ”N2£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
![]()
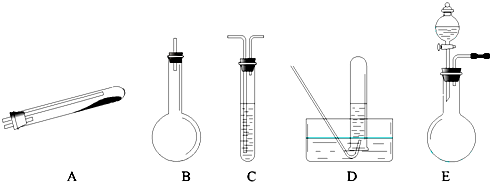
°“ÉĻŹöĶ¾¾¶ÖĘN2æɹ©Ń”ŌńµÄŹµŃéŅĒĘ÷ČēĻĀĶ¼ĖłŹ¾£¬ĘäĖū±ŲŅŖµÄŅĒĘ÷ČēĢś¼ÜĢØ”¢Ģś¼Š”¢ĢśČ¦”¢ŹÆĆŽĶų”¢¾Ę¾«µĘµČĪ“ĮŠ³ö”£

£Øl£©°“Ķ¾¾¶ ¢Ł ÖĘČ”N2Ź±ĖłŠčµÄ°±ĘųŅŖÓĆÅØ°±Ė®ÓėÉśŹÆ»Ņ×÷ŌĮĻÖĘČ”£¬»Æѧ·½³ĢŹ½ĪŖ£ŗ
![]() £¬×īŗĆŅŖÓĆÉĻŹöŅĒĘ÷ÖŠ µÄ£ØĢīŅĒĘ÷×ÖÄø£¬ĻĀĶ¬£©×÷ĪŖ°±Ęų·¢Éś×°ÖĆ”£ŅŖÖĘČ”²¢ŹÕ¼Æ“æ¾»µÄN2£ØŌŹŠķŗ¬ÉŁĮæµÄĖ®ÕōĘų£©£¬»¹Ó¦Ź¹ÓƵ½ÉĻŹöŅĒĘ÷ÖŠµÄ
£¬×īŗĆŅŖÓĆÉĻŹöŅĒĘ÷ÖŠ µÄ£ØĢīŅĒĘ÷×ÖÄø£¬ĻĀĶ¬£©×÷ĪŖ°±Ęų·¢Éś×°ÖĆ”£ŅŖÖĘČ”²¢ŹÕ¼Æ“æ¾»µÄN2£ØŌŹŠķŗ¬ÉŁĮæµÄĖ®ÕōĘų£©£¬»¹Ó¦Ź¹ÓƵ½ÉĻŹöŅĒĘ÷ÖŠµÄ
£Ø2£© ¢Ł ŗĶ ¢Ś Į½ÖÖĶ¾¾¶³£³£±»ŠĶ¬Ź¹ÓĆ”£ÕāÖÖ·½·ØÓėĶ¾¾¶ ¢Ū Ļą±ČÓŵćŹĒ
£Ø3£©¼ģ²é E ×°ÖĆĘųĆÜŠŌµÄ·½·ØŹĒ ”£ĄūÓĆ E ×°ÖĆ»¹æÉŅŌÖĘČ”µÄĘųĢåÓŠ £ØŠ“³öČżÖÖ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 N2+3Cu+3H2O
N2+3Cu+3H2O NaCl+N2”ü+2H2O£®
NaCl+N2”ü+2H2O£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅæÉŅŌĶعżČżĢõĶ¾¾¶°“²»Ķ¬ŅŖĒóĄ“ÖĘČ”µŖĘų£ŗ
¢Ł¼ÓČČĢõ¼žĻĀÓĆNH3»¹ŌCuOÖĘµĆ“æ¾»µÄN2ŗĶ»īŠŌĶ·Ū£»
¢Ś½«æÕĘųĶعż×ĘČȵĻīŠŌĶ·ŪÖĘµĆ½Ļ“æ¾»µÄN2£»
¢Ū¼ÓČČNaNO2£ØŅ»ÖÖÖĀ°©ĪļÖŹ£©ÓėNH4ClµÄ»ģŗĻÅØČÜŅŗÖĘČ”N2”£
ČēĶ¼ĖłŹ¾£¬øų³öĮĖ°“ÉĻŹöĶ¾¾¶ÖĘN2µÄæɹ©Ń”ŌńµÄ¼øÖÖŹµŃéŅĒĘ÷£¬ĘäĖū±ŲŅŖµÄŅĒĘ÷ČēĢś¼ÜĢØ”¢Ģś¼Š”¢ĢśČ¦”¢ŹÆĆŽĶų”¢¾Ę¾«µĘµČĪ“ĮŠ³ö”£

£Ø1£©°“Ķ¾¾¶¢ŁÖĘČ”N2Ź±ĖłŠčµÄ°±ĘųŅŖÓĆÅØ°±Ė®ÓėÉśŹÆ»Ņ×÷ŌĮĻÖĘČ”£¬×īŗĆŅŖÓĆÉĻŹöŅĒĘ÷ÖŠµÄ £ØĢīŅĒĘ÷“śĀė£¬ĻĀĶ¬£©×÷ĪŖ°±Ęų·¢Éś×°ÖĆ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£ŅŖÖĘČ”²¢ŹÕ¼Æ“æ¾»µÄN2£ØŌŹŠķŗ¬ÉŁĮæµÄĖ®ÕōĘų£©£¬»¹Ó¦Ź¹ÓƵ½ÉĻŹöŅĒĘ÷ÖŠµÄ £Ø°“ĘųĮ÷“Ó×óµ½ÓŅµÄĖ³ŠņĮŠ³ö£©”£
£Ø2£©°“Ķ¾¾¶¢ŚÖĘČ”N2£¬ŌĮĻĘųæÕĘųŹĒĶعżĻņ £ØĢīÉĻŹöŅĒĘ÷“śĀė£©ÖŠ¼ÓČė
¶ųĖĶČė·“Ó¦Ę÷µÄ”£
£Ø3£©ÉĻŹöČżÖÖÖĘČ”N2µÄĶ¾¾¶£¬¢ŁŗĶ¢ŚĮ½ĢõĶ¾¾¶³£³£±»ŠĶ¬Ź¹ÓƶųŌ½Ą“Ō½ŹÜµ½ČĖĆĒµÄ¹Ų×¢£¬ÕāÖÖ·½·ØÓėĶ¾¾¶¢ŪĻą±ČĘäÓÅŌ½ŠŌŌŚÓŚ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com