
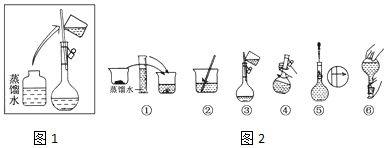
·ÖĪö £Ø1£©ŅĄ¾ŻÅäÖĘČÜŅŗĢå»żŃ”ŌńŗĻŹŹ¹ęøńČŻĮæĘæ£»ČŻĮæĘæ“ųÓŠ»īČū£¬ĪŖ·ĄÖ¹Ā©Ņŗ£¬Ź¹ÓĆĒ°ŠčŅŖ¼ģ²éŹĒ·ńĀ©Ė®£»
£Ø2£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½Öč£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ¾Ż“Ė½ā“š£»
£Ø3£©ŅĄ¾Żm=CVM¼ĘĖćČÜÖŹµÄÖŹĮ棻
£Ø4£©·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘ480mL0.5mol•L-1 NaOHČÜŅŗ£¬Ó¦Ń”Ōń500mLČŻĮæĘæ£¬ČŻĮæĘæ“ųÓŠ»īČū£¬ĪŖ·ĄÖ¹Ā©Ņŗ£¬Ź¹ÓĆĒ°ŠčŅŖ¼ģ²éŹĒ·ńĀ©Ė®£»
¹Ź“š°øĪŖ£ŗ500£»²éĀ©£»
£Ø2£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½Öč£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČ£¬Ķ¼Ź¾²Ł×÷ĪŖĻ“µÓ”¢Ņ”¶Æŗó£¬ÓĆ½ŗĶ·µĪ¹Ü¶ØČŻĒ°£¬¹ŹŃ”£ŗC£»
¹Ź“š°øĪŖ£ŗC£»
£Ø3£©ÅäÖĘ480mL0.5mol•L-1 NaOH£¬Ó¦Ń”Ōń500mLČŻĮæĘ棬ŠčŅŖČÜÖŹÖŹĮæm=0.5mol/L”Į0.5L”Į40g/mol=10.0g£»
¹Ź“š°øĪŖ£ŗ10.0g£»
£Ø4£©¢Ł¶ØČŻŹ±ø©ŹÓæĢ¶ČĻߣ¬¶¼ŌŚČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ¬
¹Ź“š°øĪŖ£ŗĘ«øߣ»
¢ŚŅ”ŌČŗó£¬ŅŗĆęµĶÓŚæĢ¶ČĻߣ¬Ć»ÓŠŌŁ¼ÓĖ®£¬ŹōÓŚÕżČ·²Ł×÷£¬ČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»ŹÜÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»ŹÜÓ°Ļģ£»
¹Ź“š°øĪŖ£ŗĪŽÓ°Ļģ£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬Ć÷Č·ÅäÖĘŌĄķ¼°²Ł×÷²½ÖčŹĒ½āĢā¹Ų¼ü£¬×¢ŅāĪó²ī·ÖĪöµÄ·½·ØŗĶ¼¼ĒÉ£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øßĆĢĖį¼Ų³ä·Ö¼ÓČČŗóµÄŹ£Óą¹ĢĢ唢µā¾Ę”¢æÕĘų¶¼ŹĒ»ģŗĻĪļ | |
| B£® | Na2OŗĶNH3µÄĖ®ČÜŅŗ¾łÄܵ¼µē£¬¹Ź¾łĪŖµē½āÖŹ | |
| C£® | Ńõ»Æ»¹Ō·“Ó¦ÖŠ·Ē½šŹōµ„ÖŹÖ»×÷Ńõ»Æ¼Į | |
| D£® | Ėį¼īÖŠŗĶ·“Ó¦µÄŹµÖŹŹĒH+ÓėOH-½įŗĻÉś³ÉĖ®£¬¹ŹĖį¼īÖŠŗĶ·“Ó¦¶¼æÉÓĆH++OH-ØTH2O±ķŹ¾ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2HgO$\frac{\underline{\;\;”÷\;\;}}{\;}$2Hg+O2”ü | B£® | Fe3O4+4CO$\frac{\underline{\;øßĪĀ\;}}{\;}$3Fe+4CO2”ü | ||
| C£® | Fe+CuSO4ØTFeSO4+Cu | D£® | 2NaCl£ØČŪČŚ£©$\frac{\underline{\;Ķصē\;}}{\;}$2Na+Cl2”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 £©µÄ¹ż³ĢÖŠ£¬ÓūŹ¹Ō×ÓĄūÓĆĀŹ“ļµ½×īøߣ¬»¹ŠčŅŖĘäĖūµÄ·“Ó¦ĪļŹĒ£Ø””””£©
£©µÄ¹ż³ĢÖŠ£¬ÓūŹ¹Ō×ÓĄūÓĆĀŹ“ļµ½×īøߣ¬»¹ŠčŅŖĘäĖūµÄ·“Ó¦ĪļŹĒ£Ø””””£©| A£® | COŗĶCH3OH | B£® | CO2ŗĶH2O | C£® | H2ŗĶCO2 | D£® | CH3OHŗĶH2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚ0”ę£¬101 kPaŹ±£¬22.4 LĒāĘųÖŠŗ¬ÓŠNAøöĒāŌ×Ó | |
| B£® | ŌŚH2O2+Cl2ØT2HCl+O2·“Ó¦ÖŠ£¬ĆæÉś³É32gŃõĘų£¬Ōņ×ŖŅĘ2NAøöµē×Ó | |
| C£® | ±ź×¼×“æöĻĀ£¬·Ö×ÓŹżĪŖNAµÄCO”¢C2H4»ģŗĻĘųĢåĢå»żŌ¼ĪŖ22.4L£¬ÖŹĮæĪŖ28g | |
| D£® | NAøöŅ»Ńõ»ÆĢ¼·Ö×ÓŗĶ0.5mol¼×ĶéµÄÖŹĮæ±ČĪŖ7£ŗ2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 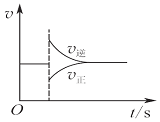 Ķ¼æÉŅŌ±ķŹ¾¶Ōij»ÆŃ§Ę½ŗāĢåĻµøıäĪĀ¶Čŗó·“Ó¦ĖŁĀŹĖꏱ¼äµÄ±ä»Æ | |
| B£® | 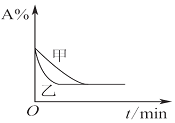 Ķ¼±ķŹ¾Ń¹Ēæ¶ŌæÉÄę·“Ó¦2A£Øg£©+2B£Øg£©?3C£Øg£©+D£Øg£©µÄÓ°Ļģ£¬ĒŅ¼×µÄŃ¹Ēæ“ó | |
| C£® | 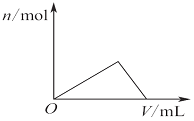 Ķ¼±ķŹ¾ĻņAl2£ØSO4£©3ŗĶMgSO4µÄ»ģŗĻŅŗÖŠµĪ¼ÓNaOHČÜŅŗ£¬Éś³É³ĮµķµÄĮæÓėµĪČėNaOHČÜŅŗĢå»żµÄ¹ŲĻµ | |
| D£® | 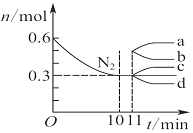 Ķ¼±ķŹ¾ŌŚ2 LµÄĆܱÕČŻĘ÷ÖŠ·¢ÉśŗĻ³É°±·“Ó¦Ź±N2µÄĪļÖŹµÄĮæĖꏱ¼äµÄ±ä»ÆĒśĻߣ¬0”«10 minÄŚøĆ·“Ó¦µÄĘ½¾łĖŁĀŹv£ØH2£©=0.045 mol•L-1•min-1£¬“Ó11 minĘšĘäĖūĢõ¼ž²»±ä£¬Ń¹ĖõČŻĘ÷µÄĢå»żĪŖ1 L£¬Ōņn£ØN2£©µÄ±ä»ÆĒśĻßĪŖd |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ńõ»ÆŠŌ£ŗB2£¾C2£¾A2 | |
| B£® | ŌŚŗ¬ÓŠC-ŗĶA-µÄČÜŅŗÖŠ¼ÓČėB2£¬C-ÓÅĻČ·¢Éś·“Ó¦ | |
| C£® | »¹ŌŠŌ£ŗA-£¾C-£¾B- | |
| D£® | ŌŚŗ¬ÓŠB2ŗĶC2µÄČÜŅŗÖŠ¼ÓČėA-£¬B2ÓÅĻČ·¢Éś·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½öŗ¬ÓŠNa+”¢H+”¢OH-”¢CH3COO-ĖÄÖÖĄė×ÓµÄijČÜŅŗÖŠæÉÄÜ“ęc£ØNa+£©£¾c£ØOH-£©£¾c£ØCH3COO-£©£¾c£ØH+£© | |
| B£® | ÅضČĻąµČµÄCH3COOHČÜŅŗÓėNaOHČÜŅŗ»ģŗĻŗóĻŌÖŠŠŌ£¬c£ØNa+£©£¾c£ØCH3COO-£©£¾c£ØOH-£©=c£ØH+£© | |
| C£® | ³£ĪĀĻĀ£¬pH=2µÄ“×ĖįÓėpH=12µÄĒāŃõ»ÆÄʵČĢå»ż»ģŗĻŗ󣬻ģŗĻŅŗÖŠc£ØNa+£©£¾c£ØCH3COO-£©£¾c£ØOH-£©£¾c£ØH+£© | |
| D£® | ½«pH=8.5µÄ°±Ė®¼ÓĖ®Ļ”ŹĶŗó£¬ČÜŅŗÖŠĖłÓŠĄė×ÓµÄÅØ¶Č¾ł½µµĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ńõ»ÆĆ¾ÓėĻ”ŃĪĖį»ģŗĻ£ŗO2-+2 H+ØTH2O | |
| B£® | Ģ¼ĖįÄĘČÜŅŗŗĶŹÆ»ŅČé·“Ó¦£ŗCO32-+Ca2+ØTCaCO3”ż | |
| C£® | Ļ”ĮņĖįÓėŠæ·“Ó¦£ŗ2 H++ZnØTZn2++H2”ü | |
| D£® | Ļņ·ŠĖ®ÖŠµĪ¼ÓFeCl3ČÜŅŗÖʱøFe£ØOH£©3½ŗĢå£ŗFe3++3H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$Fe£ØOH£©3”ż+3H+ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com