
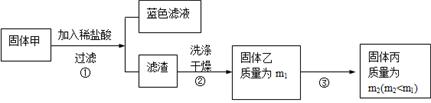
��һ��NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�������ʵ�飬
ͨ��������Ӧǰ��C��Dװ�������ı仯���ⶨ�û�����и���ֵ�����������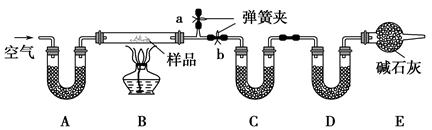
(1)����ǰͨ�������Ŀ���� ��
��������Ϊ ��
(2)װ��A��C��D��ʢ�ŵ��Լ��ֱ�Ϊ��A ��
C ��D ��
(3)����Aװ�û���ʢ��NaOH��Һ��ϴ��ƿ�����õ�NaCl�ĺ����� (�ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ)����B�з�Ӧ���Ҳ���ˮ������������ⶨ�����NaHCO3�ĺ����� ������ȥEװ�ã�����Na2CO3��10H2O�ĺ����� ��
(4)����Ʒ����Ϊw g����Ӧ��C��D���ӵ������ֱ�Ϊm1 g��m2 g���ɴ˿�֪�������NaHCO3����������Ϊ (�ú�w��m1��m2�Ĵ���ʽ��ʾ)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(14 ��)���ǵؿ��к�����ߵĽ���Ԫ�أ��䵥�ʼ��Ͻ������������е�Ӧ�������㷺��
(1)���̼�Ȼ�ԭ-�Ȼ�����ʵ�����������Ʊ�������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
Al2O3(s)+AlC13(g)+3C(s) ="3AlCl(g)+3CO(g)" ��H="a" kJ��mol��1
3AlCl(g)=2Al(l)+AlC13(g) ��H="b" kJ��mol��1
�ٷ�ӦAl2O3(s)+3C(s)=2Al(l)+3CO(g)�ġ�H= kJ��mol��1(�ú�a��b �Ĵ���ʽ��ʾ)��
��Al4C3�Ƿ�Ӧ�����е��м���Al4C3�����ᷴӦ(����֮һ�Ǻ�������ߵ���) �Ļ�ѧ����ʽΪ ��
(2)þ���Ͻ�(Mg17Al12 )��һ��DZ�ڵ�������ϣ�������������£���һ����ѧ�����ȵ�Mg��Al ������һ���¶���������á��úϽ���һ����������ȫ����ķ�Ӧ����ʽΪMg17Al122+17H2=17MgH2+12Al���õ��Ļ����Y(17MgH2 +12Al)��һ�������¿��ͷų�������
�������Ʊ�þ���Ͻ�(Mg17Al12)ʱͨ�������Ŀ���� ��
����6. 0 mol��L-1 HCl ��Һ�У������Y ����ȫ�ͷų�H2��1 mol Mg17 Al12��ȫ�����õ��Ļ����Y ������������ȫ��Ӧ���ͷų�H2�����ʵ���Ϊ ��
����0. 5 mol��L-1 NaOH ��1. 0 mol��L-1 MgCl2��Һ�У� ͼ8
�����Y ��ֻ�ܲ��ַų���������Ӧ������������ʵ�X������������ͼ��ͼ��ʾ(X����������������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ)��������NaOH ��Һ�У������Y �в�����������Ҫ������ (�ѧʽ)��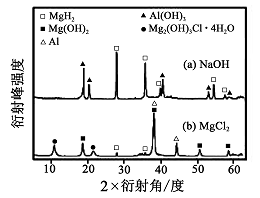
(3)�����������Խ��Al-AgO ��ؿ�����ˮ�¶�����Դ����ԭ����ͼ��ʾ���õ�ط�Ӧ�Ļ�ѧ����ʽΪ ��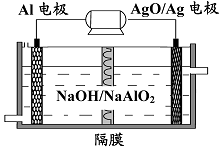
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
������Ʒ�������п�����������������Ʒ�ı�������һ�����ܵ�Fe3O4��ijѧϰС��Ϊ���о�������Ƭ���ֱ����������ʵ�������
�ٰ�һ����������Ƭ�ӹ��ɾ��ȷ�ĩ��
��ȡm g�÷�ĩ������28.00 mL 1 mol��L�������У�ǡ����ȫ��Ӧ�����ɱ�״���µ�����134.4 mL������Һ�е���KSCN��Һ������������
����ȡ���ݲ�ͬ�����ķ�ĩ���ּӼӵ���ͬ���(V)�����ʵ���Ũ�Ⱦ�Ϊl0.00 mol��L������������Һ�У���ַ�Ӧ����ȫ���ܽ⣬�йص�ʵ���������±���ʾ(����NO�������Ψһ��ԭ����)��
| ʵ����� | �� | �� | �� |
| �����ĩ����/g | 13.68 | 27.36 | 34.20 |
| ����������������״����/L | 2.912 | 5.824 | 6.720 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣�ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ��ʾ��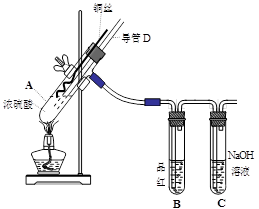
��1�� װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ�� ����Ӧ�������Թ�B�е������� ���Թ�C�������� ��
��2�� ����D���¶�(���߶�)Ӧλ�� ��Һ���ϡ�Һ���£�������D�������У���ʵ��������ų�װ���е�SO2���� ��
ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ˿���滹�к�ɫ��������ɣ����п��ܺ���CuO��Cu2O��CuS��Cu2S��Ϊ̽���ijɷ֣����������µ�ʵ�顣
�������Ͽ�֪��Cu2O + 2HCl =CuCl2+ Cu + H2O�� 2Cu2O + O2���� 4CuO��2CuS+3O2����2CuO+2SO2��Cu2S+2O2����2CuO+SO2��CuS�� Cu2S��ϡHCl����Ӧ��
|
 ��3�� �������ڿ���������ʱ��ʹ�õ�ʵ���������˲����������żܡ��ƾ����⣬�������У� ��
��3�� �������ڿ���������ʱ��ʹ�õ�ʵ���������˲����������żܡ��ƾ����⣬�������У� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�FeSO4��7H2O�㷺����ҽҩ��ҵ����������FeSO4��7H2O��ʵ�����Ʊ�����ͼ�������������������գ�
(1)��м��ϡ���ᷴӦǰ��Ӧ��10% Na2CO3��Һ���ݼ����ӣ�����Ŀ����_____�����ݺ���____����������������벢ϴ����м��
(2)����aΪ_______________��
(3)���õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ��ٳ�ȥ������渽�ŵ���������ʣ�
��____________________��
(4) FeSO4��7H2O��ijЩ��Ѫ������Ҫ�ɷ֣�ʵ����Ҫ�ⶨij��Ѫ������Ԫ�صĺ�����
I������һ������KMn04��Һ����������ԭ�ζ���������100mL 1.00 �� 10 - 2 mol��L-1��KMnO4��Һʱ�����õ���������ƽ��ҩ�ס��ձ���������������___________�����������ƣ��������ƹ����У�����˵����ȷ����____________���������ĸ����
| A��KMnO4����ˮ�����ȣ�����ֱ��������ƿ���ܽ� |
| B������ƿϴ�Ӻ�����T��ֱ������ʵ�� |
| C�����ݺ�ҡ�ȣ���Һ����ڿ̶��ߣ��ټ�ˮ����Һ����͵���̶�����ƽ |
| D���������ʱ��ˮ�����̶��߱���ع���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ʵ��С���ͬѧΪ̽�������������������ķ�Ӧ��������ͼ��ʾ��װ�ý���ʵ�顣ͨ��SO2���壬�������ǵ�ľ�������Թ�C�е���Һ�Ϸ���ľ����ȼ��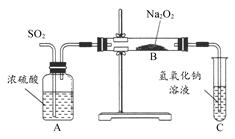
��ش��������⣺
(1)��1С��ͬѧ��ΪNa2O2��SO2��Ӧ������Na2SO3��O2���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(2)�����һ��ʵ�鷽��֤��Na2O2��SO2��Ӧ���ɵİ�ɫ�����к���Na2SO3�� ��
(3)��2С��ͬѧ��ΪNa2O2��SO2��Ӧ��������Na2SO3��O2�⣬����Na2SO4���ɡ�Ϊ�����Ƿ���Na2SO4���ɣ�������������·�����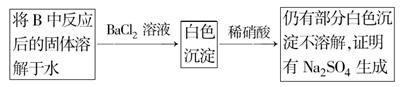
���������Ƿ������ �����Ҫ˵���������ɣ��� ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�̷���FeSO4?7H2O��������ȱ����ƶѪ����Чҩ��ijѧУ�Ļ�ѧ��ȤС���ͬѧ���̷����������µ�̽����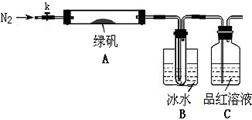
FeSO4?7H2O���Ʊ�
�û�ѧ��ȤС���ͬѧ��ʵ����ͨ������ʵ���ɷ���м������������ͭ�������������ʣ��Ʊ�FeSO4��7H2O���壺
�ٽ�5%Na2CO3��Һ���뵽ʢ��һ��������м���ձ��У����������ӣ�����������ȥ
Na2CO3��Һ��Ȼ����м��ˮϴ��2��3�顣
����ϴ�ӹ��ķ���м�м��������ϡ���ᣬ�����¶���50��80��֮������м�ľ���
�۳��ȹ��ˣ�����Һת�뵽�ܱ������У����á���ȴ�ᾧ��
�ܴ��ᾧ��Ϻ��˳����壬��������ˮϴ��2��3�Σ�������ֽ���������ɣ�
�ݽ��Ƶõ�FeSO4��7H2O�������һ��С���ƿ�У��ܱձ��档
��ش��������⣺
��1��ʵ�鲽��ٵ�Ŀ��������������������������������
��2��ʵ�鲽������Բ���������������������������������������
��3��Ϊ��ϴ�ӳ�ȥ������渽�ŵ���������ʣ�ʵ�鲽�������������ˮϴ�Ӿ��壬ԭ������������������������������������������ ��
������̽���̷���FeSO4��7H2O���ȷֽ�IJ���
��֪SO3���۵���16.8��C���е���44.8��C����С���������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���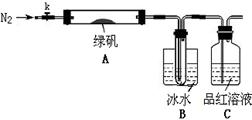
��ʵ����̡�
���������Ӻ��װ��A��B�����ԣ�
��ȡһ�����̷���������A�У�ͨ��N2������װ���ڵĿ������ر�k���þƾ��Ƽ���Ӳ�ʲ����ܣ�
�۹۲쵽A �й��������ɫ��B���Թ��ռ�����ɫҺ�壬C����Һ��ɫ��
�ܴ�A�з�Ӧ��ȫ����ȴ�����º�ȡ������Ӧ��������Թ��У����������ܽ⣬ȡ�������뼸��KSCN��Һ����Һ���ɫ��
����Bװ�õ��Թ��е��뼸��BaCl2��Һ����Һ����ǡ�
(4��ʵ��������
����1��B���ռ�����Һ���� ��
����2��C����Һ��ɫ������֪�������� ��
����3���ۺϷ�������ʵ��ۺܿ͢���֪�������һ����Fe2O3��
��ʵ�鷴˼��
��5����ָ����С����Ƶ�ʵ��װ�õ����Բ��㣺 ��
��6���ֽ��Ĺ����п��ܺ�������FeO��ȡ����ʵ����������ܽ�����Һ�������Թ��У�ѡ��һ���Լ����𣬸��Լ�����ʵ��� ��
a����ˮ��KSCN��Һ b������KMnO4��Һ c��H2O2 d��NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������Ҫ�������Σ���ũҵ������ũҩ����Ҫ��С����벡���������������ݼ����ڹ�ҵ������Ⱦɫ����������īˮ��ľ�ķ����ȡ�
��1�����Ƶ��̷���FeSO4��7H2O����dz��ɫ�ģ����ڿ����м��ױ�ɻ�ɫ������ɫ�ļ�ʽ������[Fe(OH)SO4]��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����֪FeSO4�ڲ�ͬ�����·ֽ�õ����ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��
SO3�۵���16.8�棬�е���44.8�档
ij�о���ѧϰС����������װ�ý���ʵ��̽�����ڼ���������FeSO4�ķֽ�����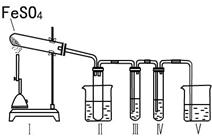
����װ�â�͢����������������Իش��������⣺
�٢�װ���ձ���ˮ���¶�Ӧ������ ��ѡ�0�桢25�桢50�桱����װ�â�������� ��
��װ�â��е��Լ������� ��ѡ����ţ���ͬ���������� ����֤����������к���SO2�� װ�â��е��Լ������� ��
| A��2 mol/LNa2CO3��Һ |
| B��Ʒ����Һ |
| C��0.5 mol/LBaCl2��Һ |
| D��0.5 mol/LBa(NO3)2 |
| �������� | Ԥ��ʵ������ | Ԥ��ʵ����� |
| ������һ����Һ�м��� �� | | �����к���Fe2O3 |
| ����һ����Һ�еμ�2�λ�ɫK3[Fe(CN)6]��Һ�� | ������ɫ���� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
С����ϲ����ѧʵ��Σ�����Ҫѧϰ��̽�������仯����������Ի�ԭ�ԡ���������һ���߽����Ļ�ѧ���á�
��1����ǰ��ʦ����������Ԥϰ��ҵ������һ����ɣ�
������ͬ��̬�����ʸ�дһ��(�������Ԫ�صĻ��ϼ�)��_____��_______�� ________��
��д��һ������֮���ת��(�����ּ�̬)�Ļ�ѧ����ʽ��___________________ ��
��2��ʵ�����ṩ�������Լ���п�������ۡ�0.1 mol��L��1 FeCl3��Һ��0.1 mol��L��1 FeCl2��Һ��KSCN��Һ��������ˮ��̽��Fe2����Fe3���������ԡ���ԭ�ԡ�
����������ԭ��Ӧ���й�ԭ����С��˵Fe2�����л�ԭ�����������ԣ�Ϊ֤ʵ�Լ��ļ��裬�����С��һ�����ʵ�鷽��������ʵ�鲢����ʵ������������б���
| ̽������ | ʵ�鷽�� | ʵ������ |
| ̽��Fe2�����л�ԭ�� | ȡ����0.1 mol��L��1 FeCl2��Һ����������__________��������Һ�м�������__________ | ��Һ���Ѫ��ɫ |
| ̽��Fe2������������ | ȡ����0.1 mol��L��1 FeCl2�� Һ������_________��� ��Ӧ | ��Һ��dz��ɫ����ɫ ��������Ӧ���ӷ���ʽΪ________________ [��Դ:Z|xx|k.Com] |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com