
| �ζ���� | ������Һ�������λ��mL�� | �����������������λ��mL�� | ||
| �ζ�ǰ���� | �ζ������ | ����������� | ||
| 1 | 20.00 | 0.50 | 20.60 | V��ƽ��= ________ |
| 2 | 20.00 | 6.00 | 26.00 | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
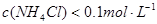 ʱ����Һ��pH
ʱ����Һ��pH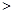 5.1������
5.1������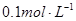 ����ζ�20mL
����ζ�20mL 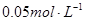 ��ˮ���ü�����ָʾ�����ﵽ�յ�ʱ������������ǣ� ��
��ˮ���ü�����ָʾ�����ﵽ�յ�ʱ������������ǣ� ��| A��10mL | B��5mL | C������10mL | D��С��5mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �¶�/�� | 25 | t1 | t2 |
| Kw/ mol2��L-2 | 1��10-14 | a | 1��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 H3COOH
H3COOH  .CH3COOH��NaOH�Ļ����Һ�Լ��ԣ�����Һ�и�����Ũ�ȴ�С����һ��Ϊ
.CH3COOH��NaOH�Ļ����Һ�Լ��ԣ�����Һ�и�����Ũ�ȴ�С����һ��Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ú��ʯ�͡���Ȼ����Ϊ��ʯ��Դ |
| B����ʯȼ����ȼ�չ������ܲ�����Ⱦ������SO2���к����� |
| C��ֱ��ȼ��ú���罫ú������ӹ�����ȼ��Ч���� |
| D����ʯ��Դ�ǿ�������Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͬʱ���з��Ӻ����ӵĵ������Һ��һ�������������Һ |
| B��pH=3�������У���c(H+)��pH=1�������е�3�� |
C��0.1 mol/L K OH��Һ��0.1 mol/L ��ˮ�У���c(OH��)��� OH��Һ��0.1 mol/L ��ˮ�У���c(OH��)��� |
D������ʱ��p H=3�������pH=11�İ�ˮ�������ϣ������Һ��pH>7 H=3�������pH=11�İ�ˮ�������ϣ������Һ��pH>7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ʱ���ų�445KJ�������������Ȼ�ѧ����ʽ��ȷ����
ʱ���ų�445KJ�������������Ȼ�ѧ����ʽ��ȷ���� g)= 1/2CO2(g)
g)= 1/2CO2(g)  +H2O(l) ��H=-890KJ/mol
+H2O(l) ��H=-890KJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ܡ����ܾ�������Ⱦ�ĸ�Ч��Դ |
| B�����������ֲ�����Ȼ����Ϊȼ�Ͽɼ��ٶԴ�������Ⱦ |
| C����úת��ΪҺ̬ȼ�Ͽ����ú��ȼ��Ч�� |
| D��ʹ�ô��������������Ӱٷ��������Է�Ӧ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��1
��1�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com