
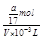 =
=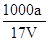 mol/L����D��ȷ��
mol/L����D��ȷ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
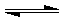 pD��g��+qE��s������H��0��m��n��p��qΪ��������ȣ���
pD��g��+qE��s������H��0��m��n��p��qΪ��������ȣ���


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 27 |
| 1 |
| 27 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
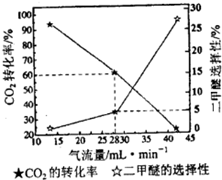
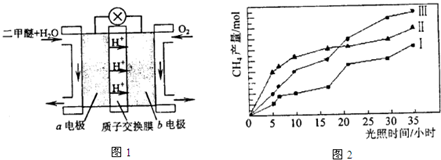
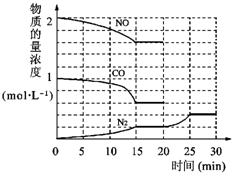 �������ⱸ�������ע���������Լ�����β���ŷŵ�һ����̼��CO�����������NOx������������强��Ϊ������Ⱦ����Ҫ���أ�
�������ⱸ�������ע���������Լ�����β���ŷŵ�һ����̼��CO�����������NOx������������强��Ϊ������Ⱦ����Ҫ���أ� O2+Hb?CO
O2+Hb?CO N2��g��+2CO2��g����H=-113kJ?mol-1
N2��g��+2CO2��g����H=-113kJ?mol-1| 0.4 |
| 15 |
| 0.4 |
| 15 |
 H2��g��+CO2��g����ƽ�ⳣ�����¶ȵı仯���±���
H2��g��+CO2��g����ƽ�ⳣ�����¶ȵı仯���±���| �¶�/�� | 400 | 500 | 830 | 1000 |
| ƽ�ⳣ��K | 10 | 9 | 1 | 0.6 |
| A | B | C | D | |
| n��CO2�� | 3 | 1 | 0 | 1 |
| n��H2�� | 2 | 1 | 0 | 1 |
| n��CO�� | 1 | 2 | 3 | 0.5 |
| n��H2O�� | 5 | 2 | 3 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com