
·ÖĪö £Ø1£©FeCl3 ŹĒĒæĖįČõ¼īŃĪ£¬Ė®½āĻŌĖįŠŌ£»
£Ø2£©¢ŁÄ³ČÜŅŗÖŠÖ»“ęŌŚOH-”¢H+”¢Na+”¢CH3COO-ĖÄÖÖĄė×Ó£¬Ė®ČÜŅŗÖŠ¶¼“ęŌŚÄ³ČÜŅŗÖŠÖ»“ęŌŚOH-”¢H+£¬Čē¹ūČÜŅŗÖŠČÜÖŹŹĒŅ»ÖÖ£¬Ö»ÄÜŹĒ“×ĖįÄĘ£»
¢ŚČōČÜŅŗÖŠĖÄÖÖĄė×ӵēóŠ”Ė³ŠņĪŖc£ØNa+£©£¾c£ØOH-£©£¾c£ØCH3COO-£©£¾c£ØH+£©£¬ČÜŅŗ³Ź¼īŠŌ£¬ĒŅc£ØOH-£©£¾c£ØCH3COO-£©£¬ĖµĆ÷ČÜŅŗ¼īŠŌŗÜĒ棬ČÜÖŹĪŖNaOHŗĶ“×ĖįÄĘ£»
¢ŪČōČÜŅŗÖŠc£ØNa+£©=c£ØCH3COO-£©£¬øł¾ŻµēŗÉŹŲŗćµĆc£ØOH-£©=c£ØH+£©£¬“×ĖįÄĘČÜŅŗ³Ź¼īŠŌ£¬ŅŖŹ¹»ģŗĻČÜŅŗ³ŹÖŠŠŌ£¬Ōņ“×ĖįµÄĪļÖŹµÄĮæÓ¦øĆÉŌĪ¢“óŠ©£®
£Ø3£©Ģ¼ĖįÄĘĖ®½āĻŌ¼īŠŌ£¬ĒŅµŚŅ»²½Ė®½ā³Ģ¶Č“óÓŚµŚ¶ž²½Ė®½ā£»½įŗĻŃōĄė×ÓĖł“ųµēŗɵČÓŚŅõĄė×ÓĖł“ųµēŗÉÅŠ¶Ļ£®
½ā“š ½ā£ŗ£Ø1£©FeCl3 ŹĒĒæĖįČõ¼īŃĪ£¬Fe3+Ė®½āĻŌĖįŠŌ£ŗFe3++3H2O?Fe£ØOH£©3+3H+£¬¹Ź“š°øĪŖ£ŗFe3++3H2O?Fe£ØOH£©3+3H+£»
£Ø2£©¢ŁÄ³ČÜŅŗÖŠÖ»“ęŌŚOH-”¢H+”¢Na+”¢CH3COO-ĖÄÖÖĄė×Ó£¬Ė®ČÜŅŗÖŠ¶¼“ęŌŚÄ³ČÜŅŗÖŠÖ»“ęŌŚOH-”¢H+£¬Čē¹ūČÜŅŗÖŠČÜÖŹŹĒŅ»ÖÖ£¬Ö»ÄÜŹĒCH3COONa£¬“×ĖįøłĄė×ÓĖ®½āµ¼ÖĀČÜŅŗ³Ź¼īŠŌ£¬µ«Ė®½ā³Ģ¶Č½ĻŠ”£¬Ąė×ÓÅØ¶Č“óŠ”Ė³ŠņŹĒc£ØNa+£©£¾c£ØCH3COO-£©£¾c£ØOH-£©£¾c£ØH+£©£¬¹Ź“š°øĪŖ£ŗc£ØNa+£©£¾c£ØCH3COO-£©£¾c£ØOH-£©£¾c£ØH+£©£»
¢ŚČōČÜŅŗÖŠĖÄÖÖĄė×ӵēóŠ”Ė³ŠņĪŖc£ØNa+£©£¾c£ØOH-£©£¾c£ØCH3COO-£©£¾c£ØH+£©£¬ČÜŅŗ³Ź¼īŠŌ£¬ĒŅc£ØOH-£©£¾c£ØCH3COO-£©£¬ĖµĆ÷ČÜŅŗ¼īŠŌŗÜĒ棬ČÜÖŹĪŖNaOHŗĶCH3COONa£¬¹Ź“š°øĪŖ£ŗNaOHŗĶCH3COONa£»
¢ŪČōČÜŅŗÖŠc£ØNa+£©=c£ØCH3COO-£©£¬øł¾ŻµēŗÉŹŲŗćµĆc£ØOH-£©=c£ØH+£©£¬“×ĖįÄĘČÜŅŗ³Ź¼īŠŌ£¬ŅŖŹ¹»ģŗĻČÜŅŗ³ŹÖŠŠŌ£¬Ōņ“×ĖįµÄĪļÖŹµÄĮæÓ¦øĆÉŌĪ¢“óŠ©£¬ŅņĪŖ¶žÕßĢå»żĻąµČ£¬ĖłŅŌ»ģŗĻĒ°c£ØNaOH£©£¼c£ØCH3COOH£©£¬
¹Ź“š°øĪŖ£ŗ£¼£»
£Ø3£©0.1mol•L-1Ģ¼ĖįÄĘČÜŅŗÖŠĢ¼ĖįøłĄė×ÓĖ®½āµ¼ÖĀČÜŅŗĻŌŹ¾¼īŠŌ£¬Ąė×ÓÅØ¶Č“óŠ”¹ŲĻµŹĒ£ŗc£ØNa+£©£¾c£ØCO32£©£¾c£ØOH-£©£¾c£ØHCO3-£©£¾c£ØH+£©£»
ŃōĄė×ÓĖł“ųµÄÕżµēŗÉ×ÜŹżµČÓŚŅõĄė×ÓĖł“ųµÄøŗµēŗÉ×ÜŹż£¬ČÜŅŗNa2CO3ÖŠµēŗÉŹŲŗć¹ŲĻµĪŖ£ŗc£ØNa+£©+c£ØH+£©=c£ØOH-£©+2c£ØCO32-£©+c£ØHCO3-£©£¬¹Ź“š°øĪŖ£ŗc£ØNa+£©£¾c£ØCO32£©£¾c£ØOH-£©£¾c£ØHCO3-£©£¾c£ØH+£©£»c£ØNa+£©+c£ØH+£©=c£ØOH-£©+2c£ØCO32-£©+c£ØHCO3-£©£®
µćĘĄ ±¾Ģāæ¼²éĄė×ÓÅØ¶Č“óŠ”±Č½Ļ”¢ŃĪĄąĖ®½ā”¢Čõµē½āÖŹµÄµēĄėµČÖŖŹ¶µć£¬²ąÖŲæ¼²é»ł±¾ŌĄķ£¬ČÜŅŗĢå»żĖį¼īŠŌ”¢Ąė×ÓÅØ¶Č“óŠ”±Č½Ļ”¢µē½āÖŹĄąŠĶ½ųŠŠ·ÖĪö½ā“š£¬ÄѵćŹĒ£Ø2£©Ģā¢ŪµÄ½ā“š£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
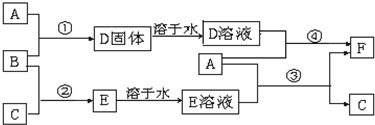
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪŽĖ®ŅŅ“¼ÓėÅØĮņĖį»ģŗĻ¼ÓČČÖĮ170”ęÉś³ÉŅŅĻ© | |
| B£® | ±½ŅŅĻ©ŌŚŗĻŹŹĢõ¼žĻĀ“߻ƼÓĒāæÉÉś³ÉŅŅ»ł»·¼ŗĶé | |
| C£® | ŅŅĻ©ÓėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·“Ӧɜ³É1£¬2-¶žäåŅŅĶé | |
| D£® | ¼×±½ÓėĀČĘųŌŚ¹āÕÕĻĀ·“Ó¦Ö÷ŅŖÉś³É2£¬4-¶žĀČ¼×±½ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | O2£¾I2£¾S | B£® | H2S£¾NaI£¾H2O | C£® | S£¾I2£¾O2 | D£® | H2O£¾NaI£¾H2S |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
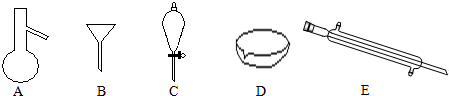
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÄĘÓėĖ®·“Ó¦£ŗNa+2H2OØTNa++2OH-+H2”ü | |
| B£® | Ļ”ĻõĖįÖŠ¼ÓČė¹żĮæĢś·Ū£ŗFe+4H++NO3-ØTFe3++NO”ü+2H2O | |
| C£® | ĀĮ·ŪĶ¶Čėµ½NaOHČÜŅŗÖŠ£ŗ2Al+2OH-ØT2AlO2-+H2”ü | |
| D£® | ĀČĘųÓėĖ®·“Ó¦£ŗCl2+H2O?H++Cl-+HClO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½šŹōÄĘÓėĖ®·“Ó¦£ŗNa+2H2OØTNa++2OH-+H2”ü | |
| B£® | ÓĆĒāŃõ»Æ±µČÜŅŗÖŠŗĶĮņĖįČÜŅŗ£ŗBa2++OH-+H++SO42-ØTH2O+BaSO4”ż | |
| C£® | ĀČ»ÆĀĮČÜŅŗÖŠ¼ÓČė¹żĮæµÄ°±Ė® Al3++4NH3•H2OØTAlO2-+4NH4++2H2O | |
| D£® | ŌŚ³ĪĒåŹÆ»ŅĖ®ÖŠĶØČė¹żĮæµÄCO2£ŗOH-+CO2ØTHCO3- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆ10mLĮæĶ²ĮæČ”7.13mLĻ”ŃĪĖį | |
| B£® | ÓĆpH¼Ę²āµĆijĻ”ŃĪĖįµÄpHĪŖ1.54 | |
| C£® | ¹ć·ŗpHŹŌÖ½ČóŹŖŗ󣬲āµĆijČÜŅŗµÄpHĪŖ2.3 | |
| D£® | ÓƱź×¼µÄŃĪĖįµĪ¶Ø“ż²āµÄNaOHČÜŅŗŹ±£¬µĪ¶ØĶź±Ļŗó£¬ĖįŹ½µĪ¶Ø¹ÜÖŠŃĪĖįµÄ¶ĮŹżĪŖ17.1mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com