
���������ε�ϡ��Һ���ֱ���a mol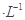 NaX��Һ��b mol
NaX��Һ��b mol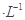 NaY��Һ������˵������ȷ����( )��
NaY��Һ������˵������ȷ����( )��
| A����a=b��pH(NaX)>pH (NaY)������ͬŨ��ʱ������HX>HY |
| B����a=b�������c��X����="c" (Y��)+c(HY)������ͬŨ��ʱ������HX>HY |
| C����a>b�����c(X��)=c(Y��)������Ƴ���Һ��c(HX)>c(HY)������ͬŨ��ʱ�� ����HX<HY |
D��������Һ�������ϣ����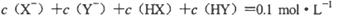 |
A
�������������A����a=b��pH(NaX)>pH (NaY)������Խ��Խˮ�����ͬŨ��ʱ������HX<HY,����B����a=b�������c��X����="c" (Y��)+c(HY)��˵��Y��ˮ��̶ȱ�X������Խ��Խˮ������ͬŨ��ʱ������HX>HY,��ȷ��C��a>b�����c(X��)=c(Y��)��˵��X��ˮ��̶ȱ�Y����������c(HX)>c(HY)������ͬŨ��ʱ�� ����HX<HY����ȷ��D������Һ�������ϣ�����a="2" [c(X��)+ c(HX) ],b="2" [c(Y��)+ c(HY) ],����a+b=2��0��1mol/L=0��2 mol/L����ȷ����ѡA��
���㣺���鲻ͬ�ε�ˮ��̶ȵĴ�С�Ƚϡ�ˮ����ɵ�Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����Һ�������25�棬�����й�������ȷ����( )
| A��PHֵ��ͬ��������Ȼ����Һ��ˮ�ĵ���̶���ͬ |
| B��ij��Һ����ˮ�������c(H+)=10-13,�����Һ��PHһ��Ϊ13 |
| C��PH=4.5�ķ���֭��c(H+)��PH=6.5��ţ����c(H+)��2�� |
| D���к�Ũ�Ⱥ��������ͬ������ʹ��ᣬ���ĵ��������Ƶ����ʵ���֮��Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
һ���¶��£����������Ƶ�����Һ�У������������ƹ����������������Ӽ��ܽ�ᾧƽ�⣺Ca(OH)2(s)  Ca2+(aq)��2OH��(aq)�����������Һ�м��������������Ʒ�ĩ����ַ�Ӧ��ָ���ԭ�¶ȡ�����������ȷ����
Ca2+(aq)��2OH��(aq)�����������Һ�м��������������Ʒ�ĩ����ַ�Ӧ��ָ���ԭ�¶ȡ�����������ȷ����
| A����Һ�и�������Ŀ��С | B����Һ�и�����Ũ�ȼ��� |
| C����Һ������������Ũ������ | D��pH��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����Һ�������25�棬�й�������ȷ����
| A��ij���ʵ���ҺpH<7���������һ�������ǿ�������� |
| B��pH=4��5�ķ���֭��c(H������pH=6��5��ţ����c��H��)��102�� |
| C������ʱ��ij��Һ����ˮ���������c(H��)��c(OH��)�ij˻�Ϊ1��10��24������Һ��һ�����Դ�������K����Na����AlO2����SO42�� |
| D������ʱ��0��l mol/L HA��Һ��pH>l��0��1 mol/L BOH��Һ��c(OH��)/c(H��)=l012������������Һ�������ϣ���Ϻ���Һ������Ũ�ȵĴ�С��ϵΪ��c��B����>c��OH����>c��H����>c��A���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������ȷ����
| A��Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ��pH�ɴ�С������˳��Ϊ��NaOH>Na2CO3>(NH4)2SO4>NaHSO4 |
| B��Ϊȷ��H2A��ǿ�ỹ�����ᣬ�ɲ�NaHA��Һ��pH����pH >7����H2A�������pH<7����H2A��ǿ�� |
| C�������£���pH=3�Ĵ�����Һϡ�͵�ԭ�����10����ϡ�ͺ���Һ��pH=4 |
| D��������KSP��AgCl����1.5��10��4����ʱ�������Ȼ�������ֱ�Ͷ����ͬ����Ģ�����ˮ��0.1mol/L�����0.1 mol/L�Ȼ�þ��Һ��0.1 mol/L��������Һ�У�����Һ��Ag��Ũ�ȣ���>��=��>�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��t��Cʱ��Ag2CrO4(�ٺ�ɫ)��ˮ��Һ�еij����ܽ�ƽ����������ͼ��ʾ��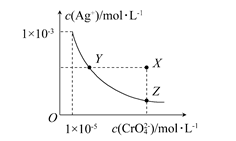
��֪AgCl��Ksp��1.8��10��10��
����˵������ȷ���� (����)
| A��t��Cʱ��Ag2CrO4��KspΪ1��10��8 |
| B���ڱ���Ag2CrO4��Һ�м���K2CrO4����ʹ��Һ��Y���ΪX�� |
| C��t��Cʱ��Y���Z���Ag2CrO4��Ksp��� |
| D��t��Cʱ����0.01 mol��L��1 AgNO3��Һ����20 mL 0.01 mol��L��1 KCl��0.01 mol��L��1 K2CrO4�Ļ����Һ�У�Cl���ȳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
25��ʱ����Ũ��Ϊ0.1000 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.1000 mol��L��1��������HX��HY��HZ���ζ���������ͼ��ʾ������˵����ȷ���ǣ�������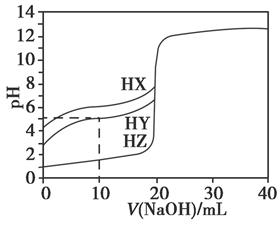
| A������ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX |
| B�����ݵζ����ߣ��ɵ�Ka��HY����10��5 |
| C��������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��c��X������c��Y������c��OH������c��H���� |
D��HY��HZ��ϣ��ﵽƽ��ʱ��c��H������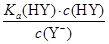 ��c��Z������c��OH���� ��c��Z������c��OH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£������е������Һ���й�˵����ȷ����(����)
| A����ͬŨ�Ⱥ������ǿ���ǿ����Һ��Ϻ���Һ��pHһ������7 |
| B����NaHCO3��Һ�У�c(CO32��)>c(HCO3��) |
| C������AgCl��������Һ�м���NaCl���壬c(Ag��)��С |
| D����pH��ȵ�CH3COONa��Na2CO3��Һϡ����ͬ������CH3COONa��Һ��pH�ϴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£�Ksp(CaSO4)��9��10��6��������CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵����ȷ���ǣ� ��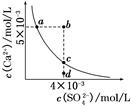
| A�����κ���Һ�У�c(Ca2��)��c(SO42-)����� |
| B��b�㽫�г������ɣ�ƽ�����Һ��c(SO42-)һ������3��10��3 mol/L |
| C��a���Ӧ��Ksp����c���Ӧ��Ksp |
| D��d����Һͨ���������Ա䵽c�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com