

| �� |
| �� |
| Mg800�� |
| ||
| ||
| ||
| ||


 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ĵ�ʡ������ѧ�ڡ�һ�ģ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
�������ѣ�TiO2���㷺Ӧ���ڸ���ṹ����Ϳ�ϡ�ֽ��Ϳ��ȣ��������ѻ�����Ϊ�Ʊ��ѵ��ʵ�ԭ�ϡ�
�������ѿ����������ַ����Ʊ���
����1��TiCl4ˮ������TiO2��xH2O�����ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2 ���˷����Ʊ��õ��������������ѡ�
��1���� TiCl4ˮ������TiO2��x H2O�Ļ�ѧ����ʽΪ_______________________________ ��
�ڼ���TiO2��x H2O��Cl���Ƿ����ķ�����______________________________
����2�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�
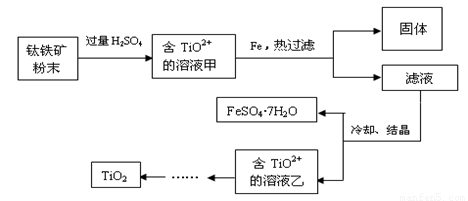
��2���������ĩ�м����ᷴӦ��TiO2+�����ӷ���ʽΪ
��3����Һ���м���Fe��������
��4����Ҫ����FeSO4��7H2O�������ˮ�������������ƾ��ơ��������⣬��Ҫ�õ������ֹ�������������
��������ѿ�������ȡ�ѵ��ʣ��漰���IJ�������ͼ��
��Ӧ�ڵķ���ʽ�� ���÷�Ӧ��Ҫ��Ar�����н��У������ԭ�� _
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�춫����ʡ���н���Э����������Ͽ������ۻ�ѧ�Ծ��������棩 ���ͣ������
�������ѹ㷺Ӧ���ڸ���ṹ����Ϳ�ϡ�ֽ��Ϳ��ȣ��������ѻ�����Ϊ�Ʊ��ѵ��ʵ�ԭ�ϡ�
�������ѿ����������ַ����Ʊ���
����1��TiCl4ˮ������TiO2��xH2O�����ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2 ���˷����Ʊ��õ��������������ѡ�
��1���� TiCl4ˮ������TiO2��x H2O�Ļ�ѧ����ʽΪ_______________________________��
�� ����TiO2��x H2O��Cl���Ƿ����ķ�����______________________________��
����2�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�
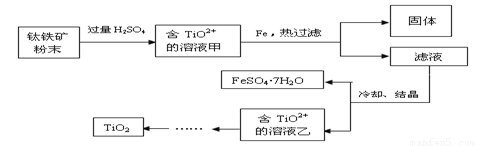
��2��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ�� ��
��3������Һ�г���TiO2+֮����еĽ����������� ��
��4����Fe�������� ��
��.�������ѿ�������ȡ�ѵ���
��5��TiO2��ȡ����Ti���漰���IJ������£�
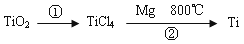
��Ӧ�ڵķ���ʽ�� ���÷�Ӧ��Ҫ��Ar�����н��У������ԭ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱���в�ƽ��������ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ������
�������ѹ㷺Ӧ���ڸ���ṹ����Ϳ�ϡ�ֽ��Ϳ��ȣ��������ѻ�����Ϊ�Ʊ��ѵ��ʵ�ԭ�ϡ�
�������ѿ����������ַ����Ʊ���
����1�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�

��1��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ��_________________________________��
��2������Һ�г���TiO2+֮����еĽ�����������__________________��
��3����Fe��������_________________________________��
����2��TiCl4ˮ������TiO2��x H2O�����ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2 ���˷����Ʊ��õ��������������ѡ�
��4���� TiCl4ˮ������TiO2��x H2O�Ļ�ѧ����ʽΪ_______________________________��
�� ����TiO2��x H2O��Cl���Ƿ����ķ�����______________________________��
�������ѿ�������ȡ�ѵ���
��5��TiO2��ȡ����Ti���漰���IJ������£�
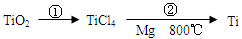
��Ӧ�ڵķ���ʽ�� ���÷�Ӧ��Ҫ��Ar�����н��У������ԭ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ�߶��ڶ�ѧ�����п��ԣ�1-3�ࣩ��ѧ���� ���ͣ������
��19�֣�����������̵���Ҫ������ͳ��õ���������������ʵ������ģ�ҵ�������̿��Ʊ�������ص�����ͼ��
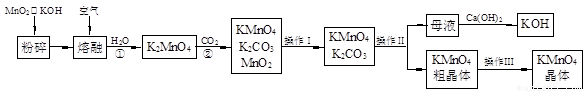
��1�������������Ϊ �������������Ϊ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ ����ҵ��������ԭ������KMnO4�������ʽϵͣ��Ϻõ��Ʊ������ǵ�ⷨ����Pt��������Fe��������K2MnO4Ϊ���Һ�������ĵ缫��ӦʽΪ ��
��3��KMnO4��һ�ֽ��ȶ��Ļ�������չ��KMnO4��Һ�ķֽ��д����ã�����MnO2��KOH��O2���� MnO2Ҳ�Ǹ÷ֽⷴӦ��һ�ִ������������һ��ʵ�鷽������֤MnO2�Ը÷ֽⷴӦ���д��ԣ� ��
��4��KMnO4�����Խ����е�ǿ�����Թ㷺Ӧ���ڷ�����ѧ�С�
���磺2KMnO4+3H2SO4+5Na2SO35Na2SO4+K2SO4+2MnSO4+3H2O��ijͬѧ��KMnO4�ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȡ�����ȷ��ȡ6.3 g Na2SO3������Ʒ�����500 mL��Һ��ȡ25.00 mL������Һ������ƿ�У���0.01000 mol/L ������KMnO4��Һ���еζ����ζ�������±���ʾ��
|
�ζ�����[��Դ:][��Դ:Z&xx&k.Com] |
������Һ�����/mL[��Դ:ѧ#��#��Z#X#X#K] |
����Һ�����[��Դ:] |
|
|
�ζ�ǰ�̶�/mL |
�ζ���̶�/mL |
||
|
1 |
25.00 mL |
0.02 |
24.01 |
|
2 |
25.00 mL |
0.70 |
24.71 |
|
3 |
25.00 mL |
0.20 |
24.20 |
������500 mLNa2SO3��Һʱ�������õ���ʵ�������У��ձ�������������ͷ�ιܡ�ҩ�� �� ��
���жϵζ��յ�������� ��
�����в����ᵼ�²ⶨ���ƫ�ߵ���
A��δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ���
B���ζ�ǰ��ƿδ����
C���ζ�ǰ�ζ��ܼ��첿��������
D���۲����ʱ���ζ�ǰ���ӣ��ζ�����
��������ʵ�����ݣ�����Na2SO3�Ĵ���Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com