
��10�֣�����ѡ����ʳ����ȷʹ��ҩ����������õ�����ϰ�ߣ��DZ�֤���Ľ�������Ҫ���棬����Щ���벻����ѧ��
��1���������� ����Ԫ�ط��ţ�����Ԫ����ɵ�һ���л������������������������Ҫ�Ĺ������ʣ��������������������Ļ�ѧ����ʽ��
��
��2�����ۡ���ά�غ� ��������Ȼ�߷��ӻ����
��3���ɼ�����۲��ַ���ˮ����Լ��� ������ĸ����
a����ˮ b���⻯����Һ c������������Һ��������Һ
��4������ѡ��ʳƷ���Ե���������Һ�����ƽ�⣬���������� ������ԡ����ԡ�����ͬ��ʳƷ�����ߡ�ˮ������ ʳƷ��
��5����֬�������������ø�������·���ˮ�⣬����Ϊ ��
��д���ƣ����������������ṩ����������Ϊ�ϳ����������������ʵ�ԭ�ϡ�
��6��������ˮ������� ��д���ƣ�����ˮ�������һ�����еĹ����������� ��____________��
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
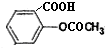 ���dz��õ�
���dz��õ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ĩ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com