
��֪�������ݣ�
|
���� |
�۵�(��) |
�е�(��) |
�ܶ�(g/cm3) |
|
�Ҵ� |
��117.3 |
78.5 |
0.789 |
|
���� |
16.6 |
117.9 |
1.05 |
|
�������� |
��83.6 |
77.5 |
0.90 |
|
Ũ����(98%) |
�� |
338.0 |
1.84 |
ѧ����ʵ������ȡ������������Ҫ�������£�
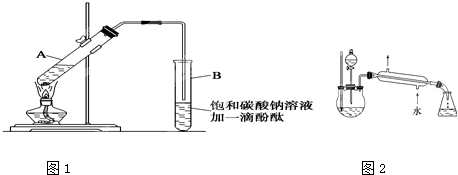
����30 mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ��(װ������������)����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10 min��

�۴��Թ�B�ռ���һ�����IJ����ֹͣ���ȣ���ȥ�Թ�B��������Ȼ���ô��ֲ㣻
�ܷ�������������㣬ϴ�ӡ����
�������ĿҪ��ش��������⣺
(1)���Ƹû����Һ����Ҫ��������Ϊ____________________________________
д����ȡ���������Ļ�ѧ����ʽ�� _____________________________________
(2)����ʵ���б���̼������Һ��������(����ĸ)_______________________________��
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
(3)���������ҪС����ȼ��Ȳ���������Ҫ������____________________________________
(4)ָ����������۲쵽������ _______________________________________________
���������������Ϊ�˸�������������ѡ�õĸ����Ϊ(����ĸ)__________��
A��P2O5 B����ˮNa2SO4 C����ʯ�� D��NaOH����
(5)ij��ѧ����С�����������ͼ��ʾ����ȡ����������װ��(ͼ�е�����̨�����С�����װ������ȥ)������ͼװ����ȣ���װ�õ���Ҫ�ŵ��У� __________________________________

(1)��һ֧30 mL�Ĵ��Թ�A��ע��4 mL�Ҵ�����������1 mL��Ũ���ᣬ�ӱ����Թܣ�����ȴ������ʱ���ټ���4 mL���Ტҡ�ȡ�CH3COOH��HOCH2CH3
CH3COOC2H5��H2O��(2)BC
(3)���ݸ����ʵķе����ݿ�֪������(117.9��)���Ҵ�(78.0��)�ķе㶼�Ƚϵͣ��������������ķе�(77.5��)�ȽϽӽ������ô����ȣ���Ӧ��������������(��������)һ��������������ԭ�ϵĴ�����ʧ����һ�����棬�¶�̫�ߣ����ܷ�����������Ӧ
(4)�Թ�B�е�Һ��ֳ��������㣬�ϲ���״Һ����ɫ(�����ŵ�ˮ����ζ)���²�Һ��(dz)��ɫ�����²�Һ��ĺ�ɫ��dz��B
(5)���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ������˸�����IJ�������������ˮ����װ�ã��������ռ����������������������˷�Һ©�������ڿ��Ʒ�Ӧ���������߷�Ӧ���������
����������1�������Ҵ���Ũ���ᡢ������Һʱ�����Լ������Թܵ�˳������Ϊ��
CH3CH2OH��Ũ�����Ũ�����CH3COOH����Ũ��������Ҵ��У��ӱ�����Ϊ�˷�ֹ���ʱ��������������Һ��ɽ�����¹ʣ����Ҵ���Ũ����Ļ��Һ��ȴ�����������ϣ���Ϊ�˷�ֹ����Ļӷ����ԭ�ϵ���ʧ���ڼ���ʱ�Թ�����ʢ��Һ���ܳ����Թ��ݻ���1/3����Ϊ�Թ��ݻ�Ϊ30 mL����ô��ʢ��Һ������10 mL���������1��4��4�ı�����Ũ���ᡢ������Ҵ��Ļ����Һ���ɴ˿�֪����Ӧ��Ũ���ᡢ������Ҵ������Ϊ1 mL��4 mL��4 mL����Ȼ�������Ѿ���������30 mL�Ĵ��Թܣ��ǾͲ��������������Թܣ��ڴ���ʱҪ�ر�ע�⡣���ƻ����Һ����Ҫ�������������Ϊ����һ֧30 mL�Ĵ��Թ�A��ע��4 mL�Ҵ�����������1 mL��Ũ���ᣬ�ӱ����Թܣ�����ȴ������ʱ���ټ���4 mL���Ტҡ�ȡ��䷴Ӧ�Ļ�ѧ����ʽΪ��
CH3COOH��HOCH2CH3 CH3COOC2H5��H2O��
CH3COOC2H5��H2O��
(2)����̼������Һ��������Ҫ��3������ʹ�������������е�������Na2CO3��Ӧ����ȥ����ʹ������Ҵ��ܽ⣻��ʹ�����������ܽ�ȼ�С����������ļ����������ķֲ���ᴿ����ѡBC�
(3)���ݸ����ʵķе����ݿ�֪������(117.9��)���Ҵ�(78.0��)�ķе㶼�Ƚϵͣ��������������ķе�(77.5��)�ȽϽӽ������ô����ȣ���Ӧ��������������(��������)һ��������������ԭ�ϵĴ�����ʧ����һ�����棬�¶�̫�ߣ����ܷ�����������Ӧ��
(4)�ڲ�����е���Ҫ�����ǣ��Թ�B�е�Һ��ֳ��������㣬�ϲ���״Һ����ɫ(�����ŵ�ˮ����ζ)���²�Һ��(dz)��ɫ�����²�Һ��ĺ�ɫ��dz������������Ǵֲ�Ʒ���������㣬���������ֲ�Ʒ���ᴿ����Ϊ������ֲ�Ʒ�м���̼���Ʒ�ĩ(Ŀ���dz�ȥ�ֲ�Ʒ�е�����)���������м��뱥��ʳ��ˮ�뱥���Ȼ�����Һ�������á���Һ(Ŀ���dz�ȥ�ֲ�Ʒ�е�̼���ơ��Ҵ�)���������м�����ˮ������(Ŀ���dz�ȥ�ֲ�Ʒ�е�ˮ)��������������������Һ�������һ���������ƿ�ڣ���������ȥ�ͷе���֣��ռ��¶���76��78��֮�����ּ��ô���������������ѡB�
(5)�Ա�����ʵ��װ��ͼ��������������Ʊ������еĸ����������ƣ����Կ������ߵ�����ͻ�����ŵ㣺���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ������˸�����IJ�������������ˮ����װ�ã��������ռ����������������������˷�Һ©�������ڿ��Ʒ�Ӧ���������߷�Ӧ��������ʡ�


 ����5��2���ϵ�д�
����5��2���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
| �� | �۵�/�� | �ۻ�����/KJ?mol-1 | �ο��۸�/Ԫ?kg-1 |
| CaCL2?6H2O | 29��0 | 37��3 | 780��850 |
| Na2SO4?10H2O | 32��4 | 77��0 | 800��900 |
| Na2HPO4?12H2O | 36��1 | 100��1 | 1600��2000 |
| Na2S2O3?5H2O | 48��5 | 49��7 | 1400��1800 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �� �� �� Ϣ |
| X | X��ij���⻯����ʹʪ��ĺ�ɫʯ����ֽ���� |
| Y | ���������õİ뵼����ϣ��㷺Ӧ���ڹ����Ϣ���� |
| Z | Z��һ�ֺ���������Ϊ27��������Ϊ14 |
| W | ����������Ӧ��ˮ������һ�ֲ�����ˮ����ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 4 |
| 1 |
| 2 |
| 1 |
| 4 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g/cm3�� |
| �Ҵ� | -117.0 | 78.0 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���ᣨ98%�� | - | 338.0 | 1.84 |
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��-859 kJ?mol-1 | B��+859 kJ?mol-1 | C��-1403 kJ?mol-1 | D��-2491 kJ?mol-1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com