
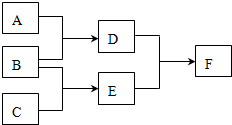
 ���� D��E��F�������ʣ�����A��B��C���ɶ�����Ԫ����ɵij������ʣ�D���Ӻ���10�����ӣ�F��һ�ֲ�������Ԫ�ص��Σ�һ��������ת����ϵ���£���ش�
���� D��E��F�������ʣ�����A��B��C���ɶ�����Ԫ����ɵij������ʣ�D���Ӻ���10�����ӣ�F��һ�ֲ�������Ԫ�ص��Σ�һ��������ת����ϵ���£���ش�
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c��NO��/mol?L-1 | 1.00��10-3 | 4.50��10-4 | 2.50��10-4 | 1.50��10-4 | 1.00��10-4 | 1.00��10-4 |
| c��CO��/mol?L-1 | 3.60��10-3 | 3.05��10-3 | 2.85��10-3 | 2.75��10-3 | 2.70��10-3 | 2.70��10-3 |
| c(NH4+) |
| c(SO42-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����֬ | B������ |
| C�������� | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
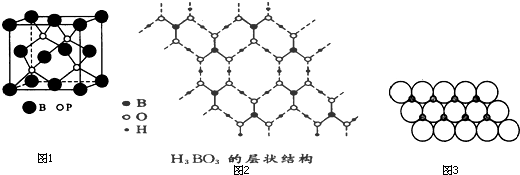
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��4�� | B��5�� | C��6�� | D��7�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CO2����ͨ��NaAlO2��Һ�� |
| B��SO2����ͨ��BaCl2��Һ�� |
| C��CO2����ͨ�뱥��Na2CO3��Һ�� |
| D��SO2����ͨ��Ba��OH��2��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢ� | C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� ���ʯģ�� ���ʯģ�� |
B�� �ɱ�ģ�� |
C��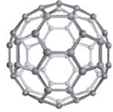 ̼60ģ�� |
D�� �Ȼ���ģ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com