
����Ŀ������ҵ�ǹ��õ���Ҫ������ҵ֮һ�����ִ����������¹�ҵ��ҽ����ҵ���й㷺Ӧ�á���ͼ�������ϳ���G��ҽ�ø߷��Ӳ���C��·��ͼ����֪B�ķ���ʽΪC6H10O3����ش��������⣺

(1)X�еĺ���������������___��X�ĺ˴Ź���������___��塣
(2)A��B�ķ�Ӧ������___��
(3)C�Ľṹ��ʽ��___��
(4)X����������Ӧ�Ļ�ѧ����ʽΪ___��
(5)д��E��F�Ļ�ѧ��Ӧ����ʽ___��
(6)A�ж���ͬ���칹�壬�������������Һ���̼̼˫���Ĺ���___��(���������칹)��
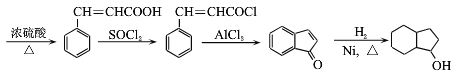
(7)��֪����![]() ��
��![]() +SOCl2��
+SOCl2��![]() +SO2+HCl���뽫������
+SO2+HCl���뽫������ Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·������ͼ��������___(���Լ�����)��
�ĺϳ�·������ͼ��������___(���Լ�����)��

![]()
���𰸡�ȩ�� 3 ������Ӧ  CH2=C(CH3)CHO+2Ag(NH3)2OH
CH2=C(CH3)CHO+2Ag(NH3)2OH![]() CH2=C(CH3)COONH4+2Ag��+3NH3+H2O
CH2=C(CH3)COONH4+2Ag��+3NH3+H2O ![]() +HCHO
+HCHO![]()
![]() +H2O 5
+H2O 5 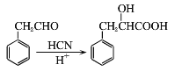
��������
����X��A�ķ�Ӧ������֪���ù���ΪX��ȩ�����������Ȼ��Ĺ��̣�����X�Ľṹ��ʽΪ![]() ��X�������ӳ�����D��̼̼˫����ȩ���е�̼��˫�����ɼӳɣ���DΪ
��X�������ӳ�����D��̼̼˫����ȩ���е�̼��˫�����ɼӳɣ���DΪ![]() ��F���Ժϳ���Ȼ������FΪ
��F���Ժϳ���Ȼ������FΪ![]() �����F��G�Ľṹ��ʽ��֪EΪ
�����F��G�Ľṹ��ʽ��֪EΪ![]() ������D��EΪ�ǻ�����ȥ��Ӧ��A���Ҷ�������������Ӧ����B������BΪ
������D��EΪ�ǻ�����ȥ��Ӧ��A���Ҷ�������������Ӧ����B������BΪ![]() ��B�к���̼̼˫���������Ӿ۷�Ӧ����CΪ
��B�к���̼̼˫���������Ӿ۷�Ӧ����CΪ ��
��
(1)XΪ![]() ���京��������Ϊȩ��������3�ֻ�������ԭ�ӣ����Ժ˴Ź���������3��壻
���京��������Ϊȩ��������3�ֻ�������ԭ�ӣ����Ժ˴Ź���������3��壻
(2)A��BΪ�Ȼ����ǻ���������Ӧ(ȡ����Ӧ)��
(3)���ݷ�����֪C�Ľṹ��ʽΪ ��
��
(4)X����������Ӧ�ķ���ʽΪCH2=C(CH3)CHO+2Ag(NH3)2OH![]() CH2=C(CH3)COONH4+2Ag��+3NH3+H2O��
CH2=C(CH3)COONH4+2Ag��+3NH3+H2O��
(5)EΪ![]() ��FΪ
��FΪ![]() �����Է�Ӧ����ʽΪ
�����Է�Ӧ����ʽΪ![]() +HCHO
+HCHO![]()
![]() +H2O��
+H2O��
(6)A��ͬ���칹�������������Һ���̼̼˫�����У�CH2=CHCOOCH3��CH2=CHCH2OOCH��CH=C(CH3)OOCH��CH3CH=CHOOCH��CH2=CHOOCCH3����5�֣�
(7)�Ա� ��
�� �Ľṹ��֪����Ҫ�������ӳɣ������γ�һ������������Ŀ������Ϣ��֪
�Ľṹ��֪����Ҫ�������ӳɣ������γ�һ������������Ŀ������Ϣ��֪![]() ���Ժ�SOCl2�γ�
���Ժ�SOCl2�γ�![]() �����ýṹ����ȡ�������ϵ���ԭ�ӣ��ݴ˿����γ���һ����״�ṹ���ٽ���ǻ����Է�����ȥ��Ӧ����˫�����ʻ����Ժ������ӳ������ǻ�����֪�ϳ�·��ӦΪ
�����ýṹ����ȡ�������ϵ���ԭ�ӣ��ݴ˿����γ���һ����״�ṹ���ٽ���ǻ����Է�����ȥ��Ӧ����˫�����ʻ����Ժ������ӳ������ǻ�����֪�ϳ�·��ӦΪ 
 ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ��Ũ�ȵ�Na2X��Һ�е������ᣬ����Ũ��������Һ��pH�仯�Ĺ�ϵ��ͼ��ʾ����֪��H2X�Ƕ�Ԫ���ᣬY��ʾ![]() ��
��![]() ��pY=��lgY�����������������
��pY=��lgY�����������������

A.����n��ʾ![]() ��pH�ı仯��ϵ
��pH�ı仯��ϵ
B.Ka1(H2X)=1.0��10��10.3
C.NaHX��Һ��c(OH��)��c(H+)
D.��pH=7ʱ�������Һ��c(Na+)=c(HX��)+2c(X2��)+c(Cl��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���FΪ��Ҫ���л�ԭ���м��壬�ϳ�F��·����ͼ��
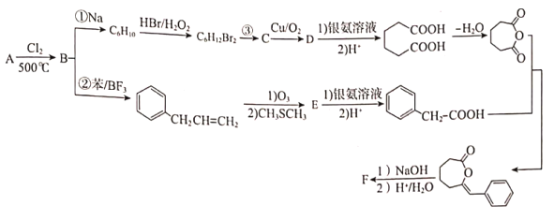
��֪��I.��A����Է�����Ϊ42��
II.R-CH=CH2![]() R-CHO+HCHO:
R-CHO+HCHO:
III.R-Cl+2Na+Cl-R'��R-R'+2NaCl
IV.��֪C=C-OH���ȶ���ԭ�ӻᷢ�����ţ�����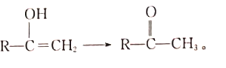
�ش��������⣺
(1)���B��������___��
(2)E�Ľṹ��ʽ��___��
(3)��Ӧ�ٵķ�Ӧ����Ϊ___����Ӧ��������Լ�������Ϊ___��
(4)��Ӧ�ڵĻ�ѧ����ʽΪ___��
(5) ��NaOH��Ӧ�Ļ�ѧ����ʽΪ___��
��NaOH��Ӧ�Ļ�ѧ����ʽΪ___��
(6)�л���![]() ��ͬ���칹���ж��֣��������ڶ�Ԫ�����ͬ���칹����___��(���������칹)д�����к˴Ź���������ʾֻ��3����ͬ���칹��Ľṹ��ʽ��___��
��ͬ���칹���ж��֣��������ڶ�Ԫ�����ͬ���칹����___��(���������칹)д�����к˴Ź���������ʾֻ��3����ͬ���칹��Ľṹ��ʽ��___��
(7)��д�����屽Ϊԭ���Ʊ�������(HOOCCH2CH2CH2CH2COOH)�ĺϳ�·��___(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ�����и���������ָ����Һ��һ���ܴ���������ǣ� ��
A.��ʹ���ȱ�����Һ�У�K����Mg2����NO3����I��
B.������Һ�У� Fe3+��Al3+��CO32-��SO42��
C.![]() =1012����Һ�У�Na+��Ba2+��Cl-��AlO2-
=1012����Һ�У�Na+��Ba2+��Cl-��AlO2-
D.c(HCO3��)��1 mol��L��1��Һ�У�Na����NH4����SO42����OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
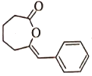
����Ŀ��25��ʱ��NaCN��Һ��CN����HCNŨ����ռ����(��)��pH�仯�Ĺ�ϵ��ͼ����ʾ����10mL0.01mol��L��1NaCN��Һ����μ���0.01mol��L��1�����ᣬ��pH�仯������ͼ����ʾ[����a�������Ϊ��9.5��0.5��]��������Һ�еĹ�ϵ����ȷ���ǣ� ��
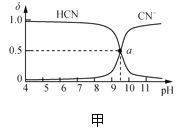
A.�����£�HCN�ĵ���ƽ�ⳣ����Ka(HCN)=10-4.5
B.ͼ����pH��7����Һ��c(Cl��)��c(HCN)
C.ͼ����b�����Һ��c(CN��)>c(Cl��)>c(HCN)>c(OH��)>c(H��)
D.ͼ����c�����Һ��c(Na��)��c(H��)��c(HCN)��c(OH��)��c(CN��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ʵ�飺��������������ͭ��Һ�� ��ͭ˿�����Ȼ�����Һ�� ��ͭ˿����ϡ�����У������ж���ȷ����( )
A.ʵ��٢�������ͭ��ϡ���ᶼֻ��������
B.ʵ��٢��з����Ķ����û���Ӧ
C.ʵ����п��ռ���NO��H2�Ļ������
D.����ʵ��֤�������ԣ�ϡ����>Cu2+>Fe2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�á��Ʊ�����TiO2�ķ���֮һ��TiCl4ˮ������TiO2xH2O�������ˡ�ˮϴ��ȥ���е�Cl-���ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2����������ԭ�ζ����ⶨTiO2������������ȡ17.2gTiO2��Ʒ��һ���������ܽⲢ��ԭΪTi3+������Һ��ˮϡ�����250mL��Һ��ȡ��25.00mL����Һ����ƿ�У��μ�KSCN��Һ��ָʾ������0.5mol/L��NH4Fe(SO4)2����Һ�ζ�Ti3+��ȫ������Ti4+����ش��������⣺
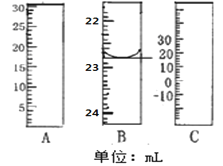
��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣�����A����������Ϊ________B����ȷ����Ϊ________��
��2��TiCl4ˮ������TiO2xH2O�Ļ�ѧ����ʽΪ________��
��3���жϵζ��յ��������_____________��
��4���ζ������յ�ʱ������ȥ0.5mol/L��NH4Fe(SO4)2����Һ40.00mL,��ԭ��Ʒ��TiO2��������______________��
��5���ж����в�����TiO2���������ⶨ�����Ӱ�죨����ƫ��������ƫ����������Ӱ��������
���������Ʊ���Һ�����У��ձ��е�NH4Fe(SO4)2��Һ������������ʹ�ⶨ���________��
�����ڵζ��յ��ȡ�ζ��ܶ���ʱ�����ӱ�ҺҺ�棬ʹ�ⶨ���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�ý���ʵ�飬��Һ��A��μ��뵽����B�У�������������ȷ����

A. ��AΪŨ���ᣬBΪKMnO4��C��ʢƷ����Һ����C����Һ��ɫ
B. ��AΪ���ᣬBΪCaCO3��C��ʢNa2SiO3����C����Һ�б����
C. ʵ������D������ֹ��Һ����������
D. ��AΪŨ��ˮ��BΪ��ʯ�ң�C��ʢAlCl3��Һ����C���Ȳ�����ɫ������������ܽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͭ��һ�ֳ���������������γ�CuSO4 .5H2O��CuSO4 .H2O�ȶ��ֽ� ��ˮ�����������ɱ�������������ڵ�ƺ͵�⾫��ͭ���մ��������⣺
(1)CuSO4 .5H2O��������________ ��
(2)��ҵ������ұ������ͭ��(��Ҫ�ɷ�CuSO4������Fe2+��AsO2-��Ca2+������)�ᴿ �Ʊ����������ͭ��(CuSO4 .H2O)�������������£�

�ܽ����õ��������˵���_____ �������IJ������б�H3AsO4��______������pHʱ���ɹ������Ҫ�ɷֳ�FeAsO4��Fe((OH)) 3���______������FeAsO4��Ӧ�����ӷ�����________,����a�辭������Ũ����____�����ˡ�ϴ�ӡ�����Ȳ��裬����ϴ�ӵĹ����г�����ˮ�Ҵ�����������______________________��
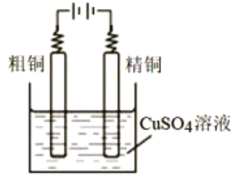
(3)��CuSO4��Һ��⾫��ͭ��װ����ͼ��ʾ����ͭ�к� п�����������ʣ����������ĵ缫��Ӧʽ_______�� Ŀǰ�������� ������չ��Cu+����������������Һ�Ʊ���ͭ���о������Cu+�� ���������CuSO4��Һ��Ƚϣ��ŵ���________________,
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com