
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B������ˮ | C���Ȼ�����Һ | D����ɫʯ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 _____________________��
_____________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �Թ�������ɫ�����ɣ� ��________________________��
�Թ�������ɫ�����ɣ� ��________________________�� ��2�����ܲ�����ɫ������������Һ�������м���BaCl2��Һ���а�ɫ�������ɵ���__________________���������ɵ���_________________��
��2�����ܲ�����ɫ������������Һ�������м���BaCl2��Һ���а�ɫ�������ɵ���__________________���������ɵ���_________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
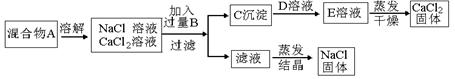
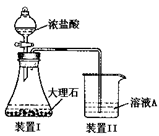
 �ӷ���ʽ�� ��
�ӷ���ʽ�� �� �������NaCl��CaCl2�������ȣ��ɳ��������C����������(��Ϊm1)�ͻ����A������(��Ϊm2)��ȷ��
�������NaCl��CaCl2�������ȣ��ɳ��������C����������(��Ϊm1)�ͻ����A������(��Ϊm2)��ȷ�� ���������NaCl��CaCl2��������Ϊ (��m1��m2��ʾ)
���������NaCl��CaCl2��������Ϊ (��m1��m2��ʾ)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
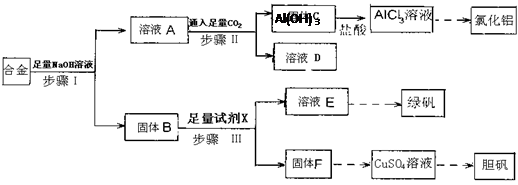
�� �ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣
�ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣 ��������ͬ����Ŀ�IJ�ͬ���ڲ�����е�Ŀ�� ���ڲ�����е�Ŀ���� ��
��������ͬ����Ŀ�IJ�ͬ���ڲ�����е�Ŀ�� ���ڲ�����е�Ŀ���� �� ���ǹ���B�� ����A��B�����㲻ѡ����һ�ֹ���������� ��
���ǹ���B�� ����A��B�����㲻ѡ����һ�ֹ���������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �и��⡣
�и��⡣�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡHCl | B��ʯ����Һ | C��ϡH2SO4 | D��BaCl2��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com