
Ė«ŃõĖ®(H2O2)ŗĶĖ®¶¼ŹĒ¼«Čõµē½āÖŹ£¬µ«H2O2±ČH2OøüĻŌĖįŠŌ”£
(1)Čō°ŃH2O2æ“³ÉŹĒ¶žŌŖČõĖį£¬ĒėŠ“³öŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½£ŗ
________________________________________________________________________
________________________________________________________________________ӣ
£Ø2£©¼ųÓŚH2O2ĻŌČõĖįŠŌ£¬ĖüÄÜĶ¬Ēæ¼ī×÷ÓĆŠĪ³ÉÕżŃĪ£¬ŌŚŅ»¶ØĢõ¼žĻĀŅ²æÉŠĪ³ÉĖįŹ½ŃĪ”£ĒėŠ“³öH2O2ÓėBa(OH)2×÷ÓĆŠĪ³ÉŃĪµÄ»Æѧ·½³ĢŹ½£ŗ
________________________________________________________________________
________________________________________________________________________ӣ
(3)Ė®µēĄėÉś³ÉH3O£«ŗĶOH£½Š×÷Ė®µÄ×ŌżµēĄė”£Ķ¬Ė®Ņ»Ńł£¬H2O2Ņ²ÓŠ¼«Ī¢ČõµÄ×ŌżµēĄė£¬Ęä×ŌżµēĄėµÄ·½³ĢŹ½ĪŖ_______________________________________________________”£
(1)H2O2
 H£«£«HO
H£«£«HO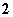 ”¢HO
ӢHO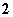

 H£«£«O
H£«£«O
(2)H2O2£«Ba(OH)2===BaO2£«2H2O»ņ2H2O2£«Ba(OH)2===Ba(HO2)2£«2H2O
(3)H2O2£«H2O2
 H3O
H3O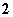 £«HO
£«HO
½āĪö””H2O2æ“³ÉŹĒ¶žŌŖČõĖį£¬µēĄė·½³ĢŹ½·Ö²½Š“£¬¼“H2O2
 H£«£«HO
H£«£«HO £¬HO
£¬HO

 H£«£«O
H£«£«O ”£H2O2£«Ba(OH)2===BaO2£«2H2O»ņ2H2O2£«Ba(OH)2===Ba(HO2)2£«2H2O”£øł¾ŻH2O£«H2O
”£H2O2£«Ba(OH)2===BaO2£«2H2O»ņ2H2O2£«Ba(OH)2===Ba(HO2)2£«2H2O”£øł¾ŻH2O£«H2O
 H3O£«£«OH£µÄ×ŌżµēĄėÖŖH2O2×ŌżµēĄėµÄ·½³ĢŹ½ĪŖH2O2£«H2O2
H3O£«£«OH£µÄ×ŌżµēĄėÖŖH2O2×ŌżµēĄėµÄ·½³ĢŹ½ĪŖH2O2£«H2O2
 H3O
H3O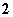 £«HO
£«HO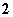 ”£
ӣ


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠ»śĪļ¼üĻߏ½½į¹¹µÄĢŲµćŹĒŅŌĻߏ¾¼ü£¬ĆæøöÕŪµćŗĶĻ߶Ė“¦±ķŹ¾ÓŠŅ»øöĢ¼Ō×Ó£¬²¢ŅŌĒā²¹×ćĖļü£¬C”¢H²»±ķŹ¾³öĄ“£¬ĘäĖüŌ×Ó»ņŌ×ÓĶÅŅŖ±ķŹ¾³öĄ“£¬ĄżČē£ŗCH3CHOHCH3µÄ¼üĻߏ½½į¹¹ĪŖ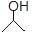 ”£ CH3CH=CHCH3ŹĒŹÆÓĶĮŃ½āµÄ²śĪļÖ®Ņ»£¬ĖüµÄ¼üĻߏ½½į¹¹æɱķŹ¾ĪŖ
”£ CH3CH=CHCH3ŹĒŹÆÓĶĮŃ½āµÄ²śĪļÖ®Ņ»£¬ĖüµÄ¼üĻߏ½½į¹¹æɱķŹ¾ĪŖ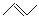 ”£
ӣ
¢ÅCH3CH=CHCH3µÄĆū³ĘĪŖ £¬ĘäĖłŗ¬¹ŁÄÜĶŵĽį¹¹Ź½ĪŖ £¬ÓėH2ŌŚŅ»¶ØĢõ¼žĻĀ·¢Éś¼Ó³É·“Ó¦£¬Ęä²śĪļµÄĶ¬·ÖŅģ¹¹ĢåµÄ¼üĻߏ½½į¹¹ĪŖ ”£
¢ĘĶź³ÉĻĀĮŠ·“Ó¦·½³ĢŹ½£¬²śĪļÓĆ¼üĻߏ½±ķŹ¾£ŗ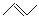 +Br2”ś £¬·“Ó¦ĄąŠĶĪŖ ·“Ó¦”£
+Br2”ś £¬·“Ó¦ĄąŠĶĪŖ ·“Ó¦”£
¢Ē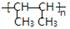 ŹĒŅ»ÖÖ¼Ó¾Ū²śĪļ£¬ŌņĘ䵄ĢåµÄ½į¹¹¼ņŹ½ĪŖ £¬ĘäĮ“½ŚĪŖ
ŹĒŅ»ÖÖ¼Ó¾Ū²śĪļ£¬ŌņĘ䵄ĢåµÄ½į¹¹¼ņŹ½ĪŖ £¬ĘäĮ“½ŚĪŖ
ӣ
¢ČŠ“³öÓėCH3CH=CHCH3ŗ¬ÓŠĻąĶ¬¹ŁÄÜĶŵÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½
”££ØČĪŠ“Ņ»ÖÖ£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®ŌŚ»Æѧ·“Ó¦¹ż³ĢÖŠ£¬·¢ÉśĪļÖŹ±ä»ÆµÄĶ¬Ź±²»Ņ»¶Ø·¢ÉśÄÜĮæ±ä»Æ
B£®ĘĘ»µ·“Ó¦²śĪļČ«²æ»Æѧ¼üĖłŠčŅŖµÄÄÜĮæ“óÓŚĘĘ»µ·“Ó¦ĪļČ«²æ»Æѧ¼üĖłŠčŅŖµÄÄÜĮæŹ±£¬·“Ó¦ĪŖĪüČČ·“Ó¦
C£®·“Ó¦²śĪļµÄ×ÜģŹ“óÓŚ·“Ó¦ĪļµÄ×ÜģŹŹ±£¬·“Ó¦ĪüČČ£¬¦¤H>0
D£®¦¤HµÄ“óŠ”ÓėČČ»Æѧ·½³ĢŹ½µÄ¼ĘĮæĻµŹżĪŽ¹Ų
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ0.1 mol”¤L£1CH3COOHČÜŅŗÖŠ“ęŌŚČēĻĀµēĄėĘ½ŗā£ŗCH3COOH
 CH3COO££«H£«£¬¶ŌÓŚøĆĘ½ŗā£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ(””””)
CH3COO££«H£«£¬¶ŌÓŚøĆĘ½ŗā£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ(””””)
A£®¼ÓČėĖ®Ź±£¬Ę½ŗāĻņÄę·“Ó¦·½ĻņŅʶÆ
B£®¼ÓČėÉŁĮæNaOH¹ĢĢå£¬Ę½ŗāĻņÕż·“Ó¦·½ĻņŅʶÆ
C£®¼ÓČėÉŁĮæ0.1 mol”¤L£1HClČÜŅŗ£¬ČÜŅŗÖŠ[H£«]¼õŠ”
D£®¼ÓČėÉŁĮæCH3COONa¹ĢĢå£¬Ę½ŗāĻņÕż·“Ó¦·½ĻņŅʶÆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«1 mol±ł“×Ėį¼ÓČėµ½Ņ»¶ØĮæµÄÕōĮóĖ®ÖŠ×īÖÕµĆµ½1 LČÜŅŗ”£ĻĀĮŠø÷ĻīÖŠ£¬±ķÕ÷ŅŃ“ļµ½µēĄėĘ½ŗāדĢ¬µÄŹĒ(””””)
A£®“×ĖįµÄÅØ¶Č“ļµ½1 mol”¤L£1
B£®[H£«]µÄÅØ¶Č“ļµ½0.5 mol”¤L£1
C£®[CH3COOH]”¢[CH3COO£]”¢[H£«]¾łĪŖ0.5 mol”¤L£1
D£®“×Ėį·Ö×ÓµēĄė³ÉĄė×ÓµÄĖŁĀŹŗĶĄė×ÓÖŲŠĀ½įŗĻ³É·Ö×ÓµÄĖŁĀŹĻąµČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĖįµÄĖįŹ½ŃĪNaHYŌŚĖ®ČÜŅŗÖŠ£¬HY-µÄµēĄė³Ģ¶ČŠ”ÓŚHY-µÄĖ®½ā³Ģ¶Č”£ÓŠ¹ŲŠšŹöÖŠÕżČ·µÄŹĒ £Ø £©
£Į.H2YŌŚµēĄėŹ±ĪŖ£ŗH2Y+H2O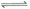 HY-+H3O+
HY-+H3O+
£Ā.ŌŚøĆŃĪµÄČÜŅŗÖŠ£¬Ąė×ÓÅضČĪŖ£ŗC(Na+)>C(Y2-)>C(HY-)>C(OH-)>C(H+)
£Ć.ŌŚøĆŃĪµÄČÜŅŗÖŠ£¬Ąė×ÓÅضČĪŖ£ŗC(Na+)>C(HY-)>C(Y2-)>C(OH-)>C(H+)
£Ä.HY-Ė®½ā·½³ĢŹ½ĪŖ£ŗHY-+H2O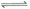 Y2-+H3O+
Y2-+H3O+
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
10 ”ꏱ¼ÓČČNaHCO3±„ŗĶČÜŅŗ£¬²āµĆøĆČÜŅŗµÄpH·¢ÉśČēĻĀ±ä»Æ£ŗ
| ĪĀ¶Č(”ę) | 10 | 20 | 30 | ¼ÓČČÖó·ŠŗóĄäČ“µ½50 ”ę |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
¼×Ķ¬Ń§ČĻĪŖ£ŗøĆČÜŅŗpHÉżøßµÄŌŅņŹĒHCO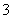 µÄĖ®½ā³Ģ¶ČŌö“󣬼īŠŌŌöĒ攣
µÄĖ®½ā³Ģ¶ČŌö“󣬼īŠŌŌöĒ攣
ŅŅĶ¬Ń§ČĻĪŖ£ŗøĆČÜŅŗpHÉżøßµÄŌŅņŹĒNaHCO3ŹÜČČ·Ö½ā£¬Éś³ÉĮĖNa2CO3£¬²¢ĶʶĻNa2CO3µÄĖ®½ā³Ģ¶Č________NaHCO3µÄĖ®½ā³Ģ¶Č(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)”£
±ūĶ¬Ń§ČĻĪŖ¼×”¢ŅŅµÄÅŠ¶Ļ¶¼²»³ä·Ö”£±ūČĻĪŖ£ŗ
(1)Ö»ŅŖŌŚ¼ÓČČÖ󷊵ÄČÜŅŗÖŠ¼ÓČė×ćĮæµÄŹŌ¼ĮX£¬Čō²śÉś³Įµķ£¬Ōņ________(Ģī”°¼×”±»ņ”°ŅŅ”±)µÄÅŠ¶ĻÕżČ·”£ŹŌ¼ĮXŹĒ________”£
A£®Ba(OH)2ČÜŅŗ B£®BaCl2ČÜŅŗ C£® NaOHČÜŅŗ D£®³ĪĒåŹÆ»ŅĖ®
(2)½«¼ÓČČÖó·ŠŗóµÄČÜŅŗĄäČ“µ½10 ”ę£¬ČōČÜŅŗµÄpH________8.3(Ģī”°øßÓŚ”±”¢”°µĶÓŚ”±»ņ”°µČÓŚ”±)£¬Ōņ¼×ÅŠ¶ĻÕżČ·”£
(3)²éŌÄ׏ĮĻ£¬·¢ĻÖNaHCO3¹ĢĢåµÄ·Ö½āĪĀ¶ČĪŖ150 ”ę£¬±ū¶ĻŃŌ________(Ģī”°¼×”±»ņ”°ŅŅ”±)ÅŠ¶ĻŹĒ“ķĪóµÄ£¬ĄķÓÉŹĒ_______________________________________________________________”£
(4)¹ŲÓŚNaHCO3±„ŗĶĖ®ČÜŅŗµÄ±ķŹöÕżČ·µÄŹĒ_______________________”£
a£®c(Na£«)£½c(HCO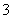 )£«c(CO
)£«c(CO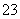 )£«c(H2CO3)
)£«c(H2CO3)
b£®c(Na£«)£«c(H£«)£½c(HCO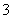 )£«c(CO
)£«c(CO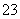 )£«c(OH£)
)£«c(OH£)
c£®HCO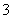 µÄµēĄė³Ģ¶Č“óÓŚHCO
µÄµēĄė³Ģ¶Č“óÓŚHCO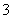 µÄĖ®½ā³Ģ¶Č
µÄĖ®½ā³Ģ¶Č
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éĄė×ÓÖŠ£¬ĆæøöĄė×Ó¶¼ÄÜÓ°ĻģĖ®µÄµēĄėĘ½ŗāµÄŅ»×éŹĒ(””””)
A£®Zn2£«”¢Ag£«”¢HCO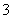 ”¢Cl£”¢PO
”¢Cl£”¢PO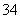 ”¢SO
”¢SO B£®Fe3£«”¢Br£”¢Al3£«”¢H£«”¢CO
B£®Fe3£«”¢Br£”¢Al3£«”¢H£«”¢CO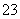 ”¢H2PO
ӢH2PO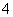
C£®Ag£«”¢SO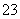 ”¢SiO
”¢SiO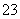 ”¢Fe2£«”¢S2£ D£®Fe2£«”¢ClO£”¢NO
”¢Fe2£«”¢S2£ D£®Fe2£«”¢ClO£”¢NO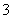 ”¢HS£”¢Cu2£«”¢HSO
”¢HS£”¢Cu2£«”¢HSO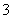
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¢ńŃŠ¾æµŖŃõ»ÆĪļÓėŠüø”ŌŚ“óĘųÖŠŗ£ŃĪĮ£×ÓµÄĻą»„×÷ÓĆŹ±£¬Ķ¬ĪĀ¶ČĻĀÉę¼°ČēĻĀ·“Ó¦£ŗ
a”¢2NO£Øg£©+Cl2£Øg£©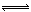 2ClNO£Øg£© ∆H1 < 0 ĘäĘ½ŗā³£ŹżĪŖK1
2ClNO£Øg£© ∆H1 < 0 ĘäĘ½ŗā³£ŹżĪŖK1
b”¢2NO2£Øg£©+NaCl£Øs£© NaNO3£Øs£©+ClNO£Øg£©∆H2 < 0 ĘäĘ½ŗā³£ŹżĪŖK2
NaNO3£Øs£©+ClNO£Øg£©∆H2 < 0 ĘäĘ½ŗā³£ŹżĪŖK2
£Ø1£©4NO2£Øg£©+2NaCl£Øs£© 2NaNO3£Øs£©+2NO£Øg£©+Cl2£Øg£© ∆H3µÄĘ½ŗā³£ŹżK= £ØÓĆK1”¢K2±ķŹ¾£©”£∆H3= £ØÓĆ∆H1”¢∆H2±ķŹ¾£©”£
2NaNO3£Øs£©+2NO£Øg£©+Cl2£Øg£© ∆H3µÄĘ½ŗā³£ŹżK= £ØÓĆK1”¢K2±ķŹ¾£©”£∆H3= £ØÓĆ∆H1”¢∆H2±ķŹ¾£©”£
£Ø2£©ĪŖŃŠ¾æ²»Ķ¬Ģõ¼ž¶Ō·“Ó¦aµÄÓ°Ļģ£¬ŌŚŗćĪĀĢõ¼žĻĀ£¬Ļņ2LŗćČŻĆܱÕČŻĘ÷ÖŠ¼ÓČė0.2mol NOŗĶ0.1mol Cl2£¬10minŹ±·“Ó¦a“ļµ½Ę½ŗā”£²āµĆ10minÄŚ¦Ō£ØClNO£©=7.5”Į10£3mol•L-1•min£1£¬ŌņĘ½ŗāŗón£ØCl2£©= mol£¬NOµÄ×Ŗ»ÆĀŹ¦Į1= ”£ĘäĖüĢõ¼ž±£³Ö²»±ä£¬·“Ó¦£Ø1£©ŌŚŗćŃ¹Ģõ¼žĻĀ½ųŠŠ£¬Ę½ŗāŹ±NOµÄ×Ŗ»ÆĀŹĪŖ¦Į2£¬¦Į1 ¦Į2£ØĢī”°>”±”°<”±»ņ”°=”±£©£¬Ę½ŗā³£ŹżK1 £ØĢī”°Ōö“ó”±”°¼õŠ””±»ņ”°²»±ä”±£©”£ČōŅŖŹ¹K1¼õŠ”£¬æɲÉÓƵēėŹ©ŹĒ ”£
IIµŚČż“ś»ģŗĻ¶ÆĮ¦³µÄæĒ°Ņ»°ćŹ¹ÓĆÄųĒāµē³Ų£¬øƵē³ŲÖŠÄųµÄ»ÆŗĻĪļĪŖÕż¼«£¬“¢Ēā½šŹō£ØŅŌM±ķŹ¾£©ĪŖøŗ¼«£¬¼īŅŗ£ØÖ÷ŅŖĪŖKOH£©ĪŖµē½āÖŹČÜŅŗ£®ÄųĒāµē³Ų³ä·ÅµēŌĄķŹ¾ŅāČēĶ¼£ŗ
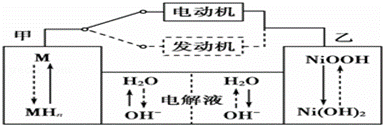
Ęä×Ü·“Ó¦Ź½ĪŖH2+2NiOOH 2Ni(OH)2 ”£øł¾ŻĖłøųŠÅĻ¢ÅŠ¶Ļ£¬»ģŗĻ¶ÆĮ¦³µÉĻĘĀ»ņ¼ÓĖŁŹ±£¬¼×µē¼«ÖÜĪ§ČÜŅŗµÄpH”” £ØĢī”°Ōö“ó”±”°¼õŠ””±»ņ”°²»±ä”±£©£¬ ŅŅµē¼«µÄµē¼«·“Ó¦Ź½”” ”””£
2Ni(OH)2 ”£øł¾ŻĖłøųŠÅĻ¢ÅŠ¶Ļ£¬»ģŗĻ¶ÆĮ¦³µÉĻĘĀ»ņ¼ÓĖŁŹ±£¬¼×µē¼«ÖÜĪ§ČÜŅŗµÄpH”” £ØĢī”°Ōö“ó”±”°¼õŠ””±»ņ”°²»±ä”±£©£¬ ŅŅµē¼«µÄµē¼«·“Ó¦Ź½”” ”””£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com