
| c(��)��V(��) |
| V(����) |
| 10��80��(V2-V1)��10-3g |
| Wg |
| 80(V2-V1)c |
| W |
| 80(V2-V1)c |
| W |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����鲡��θ�����õı��ͣ�ֻ����BaSO4����������BaCO3 |
| B��Ϊ��ʹ��ͷ�������Ƚ�ס�����ã����������Ѭ������ |
| C��������SO2��Ư��ֽ����ë��˿����ñ��� |
| D����������ˮ���ɴ����������������ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ζ�ǰ�ζ����������ݣ��ζ���������� |
| B����ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ���� |
| C���ζ�ʱ�ﵽ�ζ��յ�ʱ���Ӷ��� |
| D����ƿʢװNaOH����Һǰ������ˮϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Һ�������ǣ������� |
| B���Ҵ������ᣩ����KOH��Һ����Һ |
| C���״���Һ�����ᣩ����NaOH��Һ������ |
| D������Һ�����ͣ�����ʳ�ν��衢���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
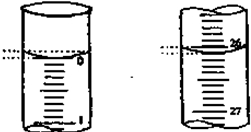
| ����� | ������������ �����/ml | 0.1000mol?L-1��������/ml | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/ml | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 34.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
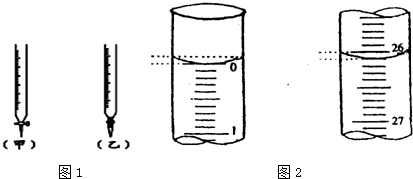
| ��ƿ����Һ | �ζ�������Һ | ָʾ�� | �ζ��� | |
| A | �� | �� | ʯ�� | ���ң� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ��̪ | ���ף� |
| D | �� | �� | ʯ�� | ���ң� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
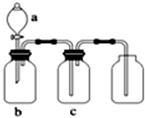 ��ͼװ�ÿ�������ȡ�����ռ������е��������壨a��b��c��ʾ��Ӧ�����м�����Լ������п��е��ǣ�������
��ͼװ�ÿ�������ȡ�����ռ������е��������壨a��b��c��ʾ��Ӧ�����м�����Լ������п��е��ǣ������� | ѡ�� | ���� | a | b | c |
| A | �������� | Ũ���� | ͭƬ | ����������Һ |
| B | �������� | Ũ���� | ͭƬ | ���Ը��������Һ |
| C | �� | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
| D | ������̼ | ϡ���� | ̼��� | Ũ���� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��FeCl2 |
| B��NaHCO3 |
| C��H2SiO3 |
| D��Fe��OH��3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com