
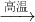 Na2S+4CO�������������� Na2SO4+4CO
Na2S+4CO�������������� Na2SO4+4CO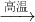 Na2S+4CO2��
Na2S+4CO2�� =
=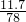 mol��������Ԫ���غ��֪����Ҫ��ˮâ�������ʵ���Ϊ
mol��������Ԫ���غ��֪����Ҫ��ˮâ�������ʵ���Ϊ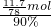 ������Ҫâ��������Ϊ
������Ҫâ��������Ϊ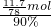 ��142g/mol=23.67g��
��142g/mol=23.67g��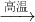 Na2S+4CO����֪������3molNa2S��Ҫ̼�����ʵ���Ϊ3mol��4=12mol�������ɵ�����ΪCO2�����ĵ�̼�����ʵ������٣����ݵ���ת���غ��֪������3molNa2S��Ҫ̼�����ʵ���Ϊ
Na2S+4CO����֪������3molNa2S��Ҫ̼�����ʵ���Ϊ3mol��4=12mol�������ɵ�����ΪCO2�����ĵ�̼�����ʵ������٣����ݵ���ת���غ��֪������3molNa2S��Ҫ̼�����ʵ���Ϊ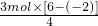 =6mol�������ĵ�̼���ʵ����ʵ��� n�ķ�Χ��6mol��n��12mol��
=6mol�������ĵ�̼���ʵ����ʵ��� n�ķ�Χ��6mol��n��12mol��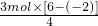 =6mol��
=6mol��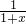 ����CO�����ʵ���Ϊ
����CO�����ʵ���Ϊ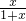 ����Ӧ���� Na2S�����ʵ���Ϊymol�����ݵ���ת���غ��У�y��[6-��-2��]=
����Ӧ���� Na2S�����ʵ���Ϊymol�����ݵ���ת���غ��У�y��[6-��-2��]=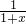 ��4+
��4+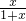 ��2��������y=
��2��������y=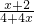 ��
��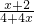 ��
��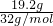 =0.6mol���ɷ���ʽ��2Na2S+Na2SO3+6HCl=NaCl+3S��+3H2O��֪���μӸ÷�Ӧ
=0.6mol���ɷ���ʽ��2Na2S+Na2SO3+6HCl=NaCl+3S��+3H2O��֪���μӸ÷�Ӧ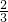 =0.4mol��Na2SO3�����ʵ���Ϊ0.6mol��
=0.4mol��Na2SO3�����ʵ���Ϊ0.6mol�� =0.2mol��
=0.2mol��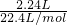 =0.1mol�����ݷ�Ӧ2HCl+Na2S=2NaCl+H2S����֪���μӸ÷�Ӧ��Na2S�����ʵ���Ϊ0.1mol������Ʒ��Na2SO4������Ϊ78.40g-0.2mol��126g/mol-��0.4mol+0.1mol����78g/mol=14.2g����Na2SO4�����ʵ���Ϊ
=0.1mol�����ݷ�Ӧ2HCl+Na2S=2NaCl+H2S����֪���μӸ÷�Ӧ��Na2S�����ʵ���Ϊ0.1mol������Ʒ��Na2SO4������Ϊ78.40g-0.2mol��126g/mol-��0.4mol+0.1mol����78g/mol=14.2g����Na2SO4�����ʵ���Ϊ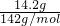 =0.1mol��
=0.1mol�� ����11.70gNa2S�����ʵ�����������Ԫ���غ������Ҫ��ˮâ�������ʵ������ٸ���m=nM������ˮâ����������
����11.70gNa2S�����ʵ�����������Ԫ���غ������Ҫ��ˮâ�������ʵ������ٸ���m=nM������ˮâ���������� ����S�����ʵ�����������������S�ķ�Ӧ�вμӷ�Ӧ��Na2S��Na2SO3���Ե����ʵ���������n=
����S�����ʵ�����������������S�ķ�Ӧ�вμӷ�Ӧ��Na2S��Na2SO3���Ե����ʵ���������n=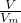 ����H2S�����ʵ������������������ᷴӦ����H2S������ҪNa2SO3�����ʵ������ٸ���m=nM������Ʒ��Na2S��Na2SO3���Ե��������̶�����Na2SO4������������n=
����H2S�����ʵ������������������ᷴӦ����H2S������ҪNa2SO3�����ʵ������ٸ���m=nM������Ʒ��Na2S��Na2SO3���Ե��������̶�����Na2SO4������������n= ����Na2SO4�����ʵ�����
����Na2SO4�����ʵ�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| x+2 |
| 4+4x |
| x+2 |
| 4+4x |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ƥ�����Ƶ���Ҫ��ѧ�Լ���������ˮâ����Na2SO4����̿���ڸ����·�Ӧ���Ƶã���Ӧʽ���£�
�� Na2SO4 + 4C ���� �� Na2S + 4CO
�� Na2SO4 + 4 CO���� �� Na2S + 4CO2
(1)��Ҫ��ȡNa2S 7.8�ˣ���Ҫ��ˮâ����Na2SO4�� g��
(2)���ڷ�Ӧ�����ɵ�Na2S���ʵ���Ϊ1 mol�������ĵ�̼���ʵ����ʵ�����Χn�ķ�Χ�� < n < ��
(3)����������Ӧ�����ĵ�̼����Ϊ1mol������Na2S�����ʵ���Ϊ y mol�����ɵ�CO��CO2�����ʵ���֮��Ϊx����y��x�Ĺ�ϵʽΪy= ��
(4)Na2S�����ڿ����У��Ỻ��������Na2SO3��Na2SO4���ֳ�ȡ�Ѿ�����������������39.2 g����ˮ�У������������ᣬ��ַ�Ӧ���˵ó���9.6g���ų�H2S����1.12L(��״��)����39.2g������������������Na2S�����ʵ���Ϊ mol��Na2SO3�����ʵ���Ϊ mol��Na2SO4�����ʵ���Ϊ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���Ϻ��г����ظ����߿�ģ�⿼�ԣ���ģ����ѧ�Ծ����������� ���ͣ�������
(��16��)��������������Ƥ�����Ƶ���Ҫ��ѧ�Լ���������ˮâ����Na2SO4����̿���ڸ����·�Ӧ���Ƶã���Ӧʽ���£�
������ Na2SO4 4C
4C Na2S
Na2S 4CO��
4CO��
������ Na2SO4 4CO
4CO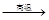 Na2S
Na2S 4CO2
4CO2
��1������Ҫ��ȡNa2S 7.80 g����������������ˮâ����Na2SO4����������Ϊ90%������������Ҫ��ˮâ����Na2SO4������������g����ȷ��0.01����
��2�������ڷ�Ӧ�����ɵ�Na2S���ʵ���Ϊ1 mol�������ĵ�̼���ʵ����ʵ���n�ķ�Χ��
���������� ��n������������ ��
��3��������������Ӧ�����ĵ�̼����Ϊ1 mol������Na2S�����ʵ���Ϊ y mol�����ɵ�CO��CO2�����ʵ���֮��Ϊx����y��x�Ĺ�ϵʽΪy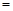 ���� ��������
���� ��������
��4����Na2S�����ڿ����У��Ỻ��������Na2SO3��Na2SO4���ֳ�ȡ�Ѿ�����������������Ʒ39.20 g����ˮ�У������������ᣬ��ַ�Ӧ���˵ó���9.6 g���ų�H2S����1.12 L����״����������㣺39.20 g��Ʒ�и��ɷֵ����ʵ����ֱ�Ϊ���� �� ����
��������д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����У�Ϻ��У�����������ʦ������У�����������ѧ�Ծ����������� ���ͣ�������
�����10�֣�
����������Ƥ�����Ƶ���Ҫ��ѧ�Լ���������ˮâ����Na2SO4����̿���ڸ����·�Ӧ���Ƶã���Ӧʽ���£�Na2SO4+ 4C Na2S+4CO�� �� Na2SO4+4CO
Na2S+4CO�� �� Na2SO4+4CO Na2S+4CO2
Na2S+4CO2
��1����Ҫ��ȡNa2S 7.80 g����������������ˮâ����Na2SO4����������Ϊ90%������������Ҫ��ˮâ����Na2SO4�� g����ȷ��0.01����
��2�����ڷ�Ӧ�����ɵ�Na2S���ʵ���Ϊ1 mol�������ĵ�̼���ʵ����ʵ���n�ķ�Χ��
�� n �� ��
��3������������Ӧ�����ĵ�̼����Ϊ1 mol������Na2S�����ʵ���Ϊy mol�����ɵ�CO��CO2�����ʵ���֮��Ϊx����y��x�Ĺ�ϵʽΪy= ��
��4��Na2S�����ڿ����У��ᱻ����������Na2SO3��Na2SO4���ֳ�ȡ�Ѿ�����������������Ʒ39.20 g����ˮ�У������������ᣬ��ַ�Ӧ����˵ó���9.6 g���ų�H2S����1.12 L����״����������㣺39.20 g��Ʒ�и�������������ʵ�����������������ˮ�е��ܽ⣩��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com