



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ѧ��ά���״�����л���ѧ�ĸ���¹���ѧ�ұ�������˹������狀ϳ������أ��״δ������л��������Ľ��� |
| B�����ϣ��ȼ�շ����ⶨ�л���������̼��Ԫ���������� |
| C���ú˴Ź������ͺ���������������Ҵ��Ͷ����ѣ�CH3OCH3�� |
| D���á�ͬλ��ʾ�ٷ������о��л���ѧ��Ӧ���̵��ֶ�֮һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��HCHO��CH3COOH | B����ϩ���Ҵ����� |
| C����ϩ�뱽��ϩ( C6H5-CH=CH2 ) | D����������ȩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
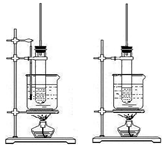
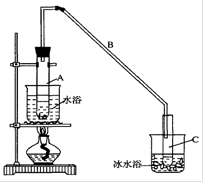
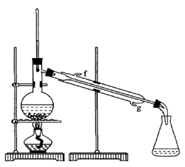
| A����ͼ������ʵ������ȡ������ |
| B����ͼ������ʵ������ȡ��ȩ��֬ |
| C���������������������������� |
| D�����������ͷ�ȩ��֬��ѡ��Ũ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B����ϩ | C������ | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֽ�����������ڷ���ͼ��黯ѧ����ʮ����������� |
| B����ֽ���������Լ����Ȼ�����Һ���Ƿ���������Ȼ�ͭ |
| C������ʱ������Һ��Ũ�Ƚϴ���ʱ������߽�С |
| D��Ϊ�˿���ɫ�ߣ�ֻ����ɫ���ӵļ���ſ�����ֽ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ۡ����� | B���٢ܡ��������� | C���ڢۢܡ����� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

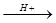 CH3CH(OH)CH2CH3 + CH3CH2CH2CH2OH
CH3CH(OH)CH2CH3 + CH3CH2CH2CH2OH 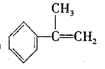 ��HBr��Ӧ����Ҫ����Ľṹ��ʽ___________________��
��HBr��Ӧ����Ҫ����Ľṹ��ʽ___________________��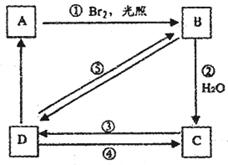
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com