
����Ŀ��������Ҫ�ɷ�Ϊ NaAlSi2O6���������������ֽ��������ӡ�
(1)NaAlSi2O6����Ԫ���У���һ��������С��Ԫ�غ͵縺������Ԫ����ɵĺ������ֻ�ѧ���Ļ����������Ϊ��______________________��
(2)Al�ĵ����Ų�ʽΪ______________________��
(3)�����K[Cr(C2O4)2(H2O)]�е������ǣ�___________��H2O������ԭ�ӵ��ӻ���������ǣ�___________����H2O��Ϊ�ȵ�����������ǣ�___________(��дһ��)
(4)Ca��O��Gr�����γ�һ�־���������Եĸ�������������ṹ��ͼ��ʾ������Ca2+��O2�����������������ܶѻ���ʽ��

�ٸþ���Ļ�ѧʽΪ��_______________��
����֪�����ӡ������Ӱ뾶�ֱ�Ϊ100pm��140pm���þ����IJ���(�߳�)Ϊ________pm��
���𰸡��������� [Ne]3s23p1��1s22s22p63s23p1 C2O42-��H2O sp3�ӻ� NH2- CaCrO3 240![]() ����
����![]() ��
��
��������
(1)����ͬ���ڴ����ҵ�һ�����ܳ��������ƣ�ͬ������ϵ��µ�һ��������С��Ԫ�طǽ�����Խǿ���縺��Խ������Խǿ���縺��ԽС��
(2)Al�ĺ��������Ϊ13�������Ų�ʽΪ[Ne]3s23p1��1s22s22p63s23p1��
(3)K[Cr(C2O4)2(H2O)]�е������ǣ�C2O42-��H2O��H2O������ԭ�ӵļ۲���Ӷ���Ϊ4������Ϊsp3�ӻ���ԭ������ͬ���۵�������ͬ�ķ��ӻ�����Ϊ�ȵ����壻
(4) �ٸ��ݾ���ṹͼ�;�̯�����㣻
������Ca2+��O2-���������������ܶѻ����м��㡣
(1) ͬ���ڴ����ҵ�һ�����ܳ��������ƣ�ͬ������ϵ��µ�һ��������С��Na��Al��Si��O����Ԫ���е�һ��������С��Ԫ����Na��Ԫ�طǽ�����Խǿ���縺��Խ������Խǿ���縺��ԽС������Na��Al��Si��O����Ԫ�ص縺���ɴ�С��˳��Ϊ��O> Si > Al > Na����NaAlSi2O6����Ԫ���У���һ��������С��Ԫ��Na�͵縺������Ԫ��O��ɵĺ������ֻ�ѧ���Ļ�������Na2O2������Ϊ�������ƣ�
��ˣ�������ȷ���ǣ��������ƣ�
(2)Al�ĺ��������Ϊ13�������Ų�ʽΪ[Ne]3s23p1��1s22s22p63s23p1��
��ˣ�������ȷ���ǣ�[Ne]3s23p1��1s22s22p63s23p1��
(3)K[Cr(C2O4)2(H2O)]�е������ǣ�C2O42-��H2O��H2O������ԭ�ӵļ۲���Ӷ���Ϊ4������Ϊsp3�ӻ���ԭ������ͬ���۵�������ͬ�ķ��ӻ�����Ϊ�ȵ����壬����H2O��Ϊ�ȵ����������Ӧ����3��ԭ�ӣ��Ҽ۵�����Ϊ8����NH2-��
��ˣ�������ȷ���ǣ�C2O42-��H2O��sp3�ӻ���NH2-��
(4) �ٸ��ݾ���ṹͼ�;�̯����֪��������Oԭ�Ӹ���Ϊ![]() 6=3��Caԭ�Ӹ���Ϊ
6=3��Caԭ�Ӹ���Ϊ![]() =1��Crԭ�Ӹ���Ϊ1����ѧʽΪCaCrO3 ��
=1��Crԭ�Ӹ���Ϊ1����ѧʽΪCaCrO3 ��
��Ca2+��O2-���������������ܶѻ�����֪�����ӡ������Ӱ뾶�ֱ�Ϊ100pm��140pm��������Խ�����480pm�����Ըþ����IJ���(�߳�)Ϊ240![]() pm����
pm����![]() pm����
pm����
��ˣ�������ȷ���ǣ�CaCrO3 ��240![]() ����
����![]() ����
����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪��P4�����ף�s����5O2��g����P4O10��s�� ��H����2983.2kJ/mol
P�����ף�s����5/4O2��g����1/4 P4O10��s�� ��H����738.5kJ/mol
�����ת��Ϊ�����Ȼ�ѧ����ʽΪ ���ɴ�˵�������ȶ��ԱȰ��� ��
��2������[KAl(SO4)2��12H2O]ˮ��Һ�� �����������������������������ԣ��������ܾ�ˮ�������ӷ���ʽ������ԭ��Ϊ ��
��3�����ص�����ת����ʽΪ ��ԭ��ط�Ӧ ������һ����������һ�������Է���
��4��pH=3�������pH=3�Ĵ�����Һ��ϡ�����������100����ϡ�ͺ������pH (��������������С��������������)�����pH������ʱpH=x�������pH=y��NaOH��Һ��1�U10������Ȼ�ϣ���Ӧ����Һǡ�ó����ԣ���x��y����Ĺ�ϵ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���10mL0.1mol/L��ijһԪ��HR��Һ����μ���0.1mol/L��ˮ��������ҺpH�����������仯��ͼ�����з�������ȷ���ǣ� ��

A. a��b�㵼��������ǿ˵��HRΪ����
B. a��b����ʾ��Һ��ˮ�ĵ���̶Ȳ���ͬ
C. bǡ����ȫ�кͣ�pH=7˵��NH4Rû��ˮ��
D. c ����Һ����c��NH4+����c��R-����c��OH-����c��H+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25������20mL 0.1mol��L-1MOH��Һ�еμ�0.1mol��L-1 CH3COOH��Һ��������Һ��pH(��Ӧ����M)����������(��Ӧ����N)�仯��ͼ��ʾ������������ȷ���ǣ� ��
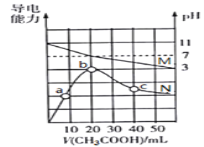
A. MOH��ǿ��
B. b����Һ�У�c(CH3COO-)��c(CH3COOH)��0.1 mol��L-1
C. ˮ�����ӻ�����Kw��b��c��a
D. c����Һ�У�c(CH3COO-)��c(M+)��c(H+)��c(OH-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��M��W������X�������ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Z ���������������ڲ��3����Y��Z�������ڣ�Z��Wλ��ͬ���壬M��Wͬ���ڣ�����Xͬ���塣
(1)MԪ����_______(��Ԫ�ط���)��
(2)Z��W�γɵ���̬�⻯���У����ȶ�����_______(�ѧʽ)��
(3)д��M2Z2�ĵ���ʽ��_______��
(4)��X��Y��Z��W����Ԫ���е�������ɵ�һ��ǿ�ᣬ��ϡ��Һ����ͭ��Ӧ�����ӷ���ʽΪ_______��
(5)��X��Y��Z��W����Ԫ����ɵ�һ�����ӻ�����A����֪��
��1molA��������NaOH�ȵ�Ũ��Һ��Ӧ���ɱ�״����44.8L���壻
��A�������ᷴӦ��������B����������ʹƷ����Һ��ɫ��
д�����з�Ӧ�Ļ�ѧ����ʽ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��U�ι���ʢ��150mL����Һ����Ҫ��ش��������⣺
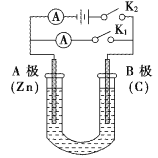
(1)��K2���պ�K1������ʢ��ҺΪϡ���ᣬ��B��Ϊ___________��(��缫����)����װ�õ�����ת����ʽΪ��__________��B���ĵ缫��ӦʽΪ��______________________��
(2)��K1���պ�K2������ʢ��ҺΪNaCl��Һ��һ��ʱ���U�ι�����Һ��pH___________(���������䡱��С��)���ܷ�Ӧ�Ļ�ѧ����ʽ�ǣ�______________________����ҪʹU�ι�����Һ�ָ�����ʼ״̬��Ӧ��U�ι��ڵ���Һ����(��ͨ��)___________��
(3)���Ҫ�������϶���ͭ����K1���պ�K2�����Һѡ��CuSO4��Һ����A�缫�IJ���Ӧ������___ (����顱��ͭ��)����Ӧһ��ʱ���������Һ��Cu2+��Ũ�Ƚ���_____ (���������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú̿��������Ϊ��ɫ��������������������ʹ�õ���Ҫ��Դ֮һ��Ϊ�����ú�������ʣ������к�������ŷţ����Dz�ȡ�˸�ʽ�����ķ�����
��1��ú��������Һ���������ú�������ʡ�ú��������������Ҫ������___________��ú��Һ�������ַ�Ϊֱ��Һ���ͼ����������ú����������ǿ�ȵõ���¯����ú���ͼ���̿�Ȳ�Ʒ�ļ�����Ϊ___________��
��2��ú��ȼ��ǰ����ȼ�չ����о��ɲ�ȡ��ʩ�����к�������ŷţ�
����ȼ��ǰ�����Բ���������������ԭ�����£�
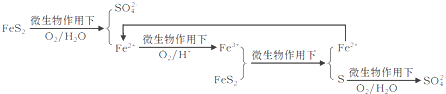
����������Fe2+������Ϊ________________��д��Fe2+![]() Fe3+�����ӷ���ʽ________________��
Fe3+�����ӷ���ʽ________________��
��ú��ȼ��ʱ��������������������________ȼ�ռ������ڰ�ú������������¯ȼ���ң�ʹú����������������г�ֻ�ϡ�ȼ�գ��������ã��������������Ҫ��ѧ�ɷ�Ϊ_______���ѧʽ����
��ú��ȼ�պ��������������ó�����������������������ʪ���������������н�����ͨ��_________�豸����ʯ��ˮ��ϴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��������ȡ���κ����±(��Ҫ�ɷ�ΪMgCl2)�Ʊ�����þ���乤���������¡�
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
����˵��������ǣ�������
A. ��ʵ����ʵʩ��������Ҫ�IJ���������©�����ձ���������
B. Mg��OH��2�D��MgCl2�����ӷ���ʽΪ��Mg��OH��2��2H��===Mg2����2H2O
C. �������ǽ�MgCl2��Һ���ɺ���ȴ�ᾧ
D. �����������������������������24��71
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������Na��Na2O��Na2O2�Ļ������������ˮ��Ӧ���ڱ�״���µõ�a L������塣���û������ͨ�������ȼ��ǡ����ȫ��Ӧ����ԭ�������Na��Na2O��Na2O2�����ʵ���֮�ȿ���Ϊ
A. 2��1��1
B. 1��1��2
C. 1��2��1
D. 4��3��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com