
����Ŀ�������ܡ����������Ƶ����ʣ��ڻ�ѧ�ϳ�Ϊ��ϵԪ�ء��ش��������⣺
��1��LiCoO2�� LiFePO4����������ӵ�ص��������ϡ���̬Coԭ�Ӻ�������Ų�ʽΪ_________�����ĵ�����I4(Co) _________I4(Fe)(����>������<��)��PO43���Ŀռ乹��Ϊ_________��
��2����ϵԪ������CO�γ�Fe(CO)5��Ni(CO)4�Ƚ����ʻ�����
��CO��Ϊ�ȵ�����ķ��Ӻ����ӷֱ�Ϊ_________��_________(����һ�֣��ѧʽ)����CO�����У�����������Ŀ֮��Ϊ_________��
��3������K2O�� (�����ϩ)�ڸ������������¹��ȿ��Ƶö�ï��[Fe(C5H5)2]���ڻ����ϩ�У�Cԭ�ӵ��ӻ��������Ϊ_________����ï���۵�Ϊ446K��������ˮ�����������ѡ������Ҵ����л��ܼ���373K���������Ӹ������ʿ������������ǵ��͵�_________�����
(�����ϩ)�ڸ������������¹��ȿ��Ƶö�ï��[Fe(C5H5)2]���ڻ����ϩ�У�Cԭ�ӵ��ӻ��������Ϊ_________����ï���۵�Ϊ446K��������ˮ�����������ѡ������Ҵ����л��ܼ���373K���������Ӹ������ʿ������������ǵ��͵�_________�����
��4�������ʵĶѻ���ʽ�����֣�������ͼ�ֱ���ͼa��b��ʾ��
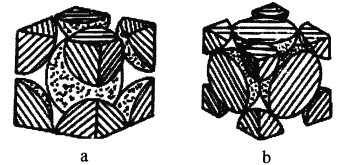
��ͼa��ʾ�ѻ���ʽ����ԭ�ӵİ뾶Ϊrpm�����侧���ⳤΪ_________cm��
��ͼb��ʾ�ѻ���ʽ����ԭ�ӵ������ռ��������ı���Ϊ_________(�ú�Բ�������Ĵ���ʽ��ʾ)
���𰸡�[Ar]3d74s2 < �������� N2 CN- 1��2 sp2��sp3 ���� ![]()
![]() ��
��
��������
��1��CoΪ27��Ԫ����ԭ�Ӻ�������Ų�ʽΪ[Ar]3d74s2��
Co3+��������Ų�Ϊ���ȶ��ṹ3d4������ʧȥ���ӣ�Fe3+��������Ų�Ϊ�����ȶ��ṹ3d5,����ʧ���ӣ��ɴ˱Ƚϵ�4�����ܣ�
PO43-��Pԭ�ӹµ��Ӷ���Ϊ0���Ҽ���ĿΪ4���ɴ�ȷ���ռ乹�ͣ�
(2)�۵�������ԭ������ȵ����ӻ�Ϊ�ȵ����壻CO��N2�Ĺ��������Ҽ����м����ֱ�Ϊ1��2��
��3���ڻ����ϩ����4��Cԭ��Ϊsp2�ӻ�,1��Cԭ��Ϊsp3�ӻ���
��ï�����۵�,�ܽ��������������������������Ӿ������ƣ�
��4����a�����ⳤΪx,4rpm=![]() x�����x����b�����ⳤΪy��Feԭ�Ӱ뾶ΪR,4R=
x�����x����b�����ⳤΪy��Feԭ�Ӱ뾶ΪR,4R=![]() y���ٽ���������ʽ���㡣
y���ٽ���������ʽ���㡣
��:��1��CoΪ27��Ԫ��,��۵����Ų�Ϊ3d74s2,��̬Coԭ�Ӻ�������Ų�ʽΪ[Ar]3d74s2��
Co3+��������Ų�Ϊ���ȶ��ṹ3d4������ʧȥ������Fe3+��������Ų�Ϊ�����ȶ��ṹ3d5,����ʧ����,��l4(C0)<l4(Fe)��
PO43-��Pԭ�ӹµ��Ӷ���Ϊ0���ļ���ĿΪ4������ռ乹��Ϊ�������壻
��2����CO�۵�������ԭ������ȵķ�����N2,������CN-,C22-����CO��N2�Ĺ�����,�Ҽ����������ֱ�Ϊ1��2���Ҽ���м���Ŀ֮��Ϊ1��2��
��3���ڻ����ϩ����4��Cԭ��Ϊsp2�ӻ�,1��Cԭ��Ϊsp3�ӻ�����ï�����۵����ܽ���������������������ʣ����������ǵ��͵Ĺ��ۻ�������
(4)��a�����ⳤΪx,4rpm=![]() x��x=
x��x=![]() rpm=
rpm=![]() r��10-10cm��
r��10-10cm��
��b�����ⳤΪy��Feԭ�Ӱ뾶ΪR,4R=![]() y����R=
y����R=![]() y���������е�ԭ����Ϊ(8��1/8+6��1/2)=4,ԭ�������Ϊ
y���������е�ԭ����Ϊ(8��1/8+6��1/2)=4,ԭ�������Ϊ![]() =
=![]() ��y3,��ԭ�ӵ������ռ��������ı���Ϊ
��y3,��ԭ�ӵ������ռ��������ı���Ϊ![]() �С�
�С�


 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��X(g)+2Y(g)![]() 3Z(g) ��H=-akJ��mol-1(a>0)������˵������ȷ����
3Z(g) ��H=-akJ��mol-1(a>0)������˵������ȷ����
A. 0.1molX��0.2molY��ַ�Ӧ����Z�����ʵ���һ��С��0.3mol
B. �ﵽ��ѧƽ��״̬ʱ��X��Y��Z��Ũ�Ȳ��ٷ����仯
C. �ﵽ��ѧƽ��״̬ʱ����Ӧ�ų����������ɴ�a Kj
D. ���߷�Ӧ�¶ȣ��淴Ӧ������������Ӧ���ʼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ba(OH)2(����)��CuSO4(����)����H2SO4(Һ̬)��Ϊһ�࣬�����������ʿ��Ժ����ǹ�Ϊһ�ࣨ ��
A.75%�ľƾ���ҺB.�Ȼ���C.ϡ����D.�ཬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�
A. 28g����ϩ�к���NA��̼̼˫��
B. 1mol��ϩ��Cl2��ȫ�ӳɣ�Ȼ����Cl2����ȡ����Ӧ�������������ķ��������Ϊ6NA
C. ��״���£�2.24LCCl4�е�ԭ����������0.5NA
D. 15g�����еĵ�������9NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3)�����������о��й㷺Ӧ�á��ش��������⣺
I.��ҵ���ձ�ʹ��Na2SO3����ǹ����Ʊ�Na2S2O3��װ����ͼ1��

��1����K1�ر�K2����Բ����ƿ�м��������Լ��ײ����ȡ��Լ���Ϊ_________��װ��B��D��������_________��
��2��ʼ�ձ���C����Һ�ʼ��ԡ����Ȳ���Na2S2O3����Ӧ�Ļ�ѧ����ʽΪ___________________________����Ӧһ��ʱ���C��������٣���ʱ��K2���ر�K1��ֹͣ���ȣ���C�����û��������ᴿ�õ�Na2S2O3��������ʱ�ر�K1��������C����Һ�����ԡ���������Ӧ����S��_________��
��.����SO2��Na2CO3��Na2S�Ļ����Һ��ӦҲ���Ʊ�Na2S2O3������������ͼ2��

��1��װ��G��Na2CO3��Na2S��������ʵ���֮��Ϊ_________��
��2�����������Ӹ��������ӿ�˳��Ϊ��_________��g��h��_________��_________��_________��_________��d��
��.����Na2S2O3��Һ�ⶨ��ˮ��Ba2+Ũ�ȡ�
ȡ��ˮ20.00mL�������ʵ�����ȼ�������K2Cr2O7��Һ���� BaCrO4����������ϴ�Ӻ�������ϡ���ܽ⣬��ʱCrO42��ȫ��ת��ΪCr2O72�����ټӹ���KI��Һ����Cr2O72����ַ�Ӧ��Cr2O72��+6I��+14H+=3I2+2Cr3++7H2O��Ȼ����������Һ��ָʾ������0.0100mol/L��Na2S2O3��Һ���еζ���I2+2S2O32��===S4O62��+2I��������Һ_________��Ϊ�յ㡣ƽ�еζ�3�Σ�����Na2S2O3��Һ��ƽ������Ϊ18.00m����÷�ˮ��Ba2+�����ʵ���Ũ��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ش��������⣺
��1��ʵ��������ʱ����һЩ�Ȼ�����Һʱ�������Ȼ������������ڽ�Ũ�������У�Ȼ����������ˮϡ�͵������Ũ�ȣ���Ϊ��_________________����ٽ����������ơ���ˮ�⡣�Ȼ���ˮ��Һ��______����ᡱ�����С���������ԣ�����ʱ��pH _____7���>������=������<������ԭ���ǣ������ӷ���ʽ��ʾ����______________________________��
��2�������£�pH=2��ij��HnA(AΪ���)��pH=12��ij��B(OH)m�������ϣ�ǡ�÷�Ӧ�������Σ������Һ��pH=10����Ӧ�������εĻ�ѧʽΪ___________ ��������___________�������ӷ��ţ�һ����ˮ�⣬��ˮ������ӷ���ʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ƚ�1mol������1molһ����̼�����������������������ڷ�����������ԭ��������������ͬ���ǣ� ��
A.��B.�٢�C.�ڢ�D.�٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ��VIAԪ�ص�����H2��Ӧ������̬H2X���Ȼ�ѧ����ʽ���£�
![]() O2(g) +H2(g) ��H2O(g)
O2(g) +H2(g) ��H2O(g) ![]() H����242kJ��mol��1
H����242kJ��mol��1
S(g)+ H2(g) ��H2S(g) ![]() H����20kJ��mol��1
H����20kJ��mol��1
Se(g)+H2(g)![]() H2Se(g)
H2Se(g) ![]() H��+81kJ��mol��1
H��+81kJ��mol��1
����˵����ȷ����
A. �ȶ��ԣ�H2O< H2S< H2Se
B. ����������Se��H2��Ӧ����H2Se
C. O2(g)+2H2S(g)��2H2O(g)+2S(g) ![]() H����444 kJ��mol��1
H����444 kJ��mol��1
D. ���ź˵���������ӣ�VIA��Ԫ�ص�����H2�Ļ��Ϸ�ӦԽ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У�����������������
A. 22.4L O2(��״��) B. 1.6 g H2
C. 1.2 mol H2SO4 D. 28g CaO
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com