



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��X��Y�γ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ֻ������ |
| B��Y��W������������������� |
| C������������Ӧˮ���������Y��W |
| D��X��Z�γɵĻ������У���ԭ�Ӿ�����8�����ȶ��ṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢� | B���ڢ� |
| C���٢ڢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
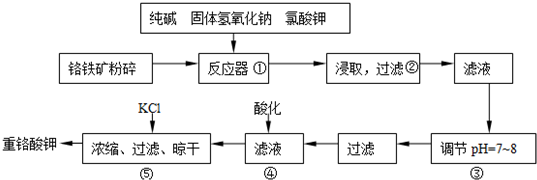
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| NaCl |
| NH4Cl |
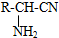 ��R-CN
��R-CN| H+ |
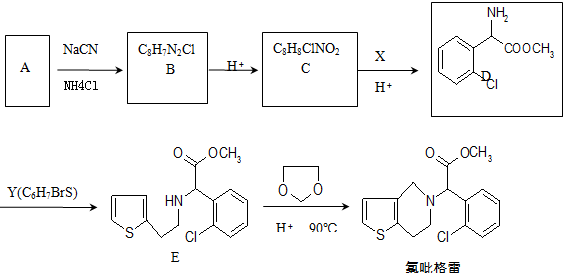
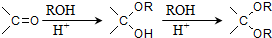 ��д������ϩ���״�Ϊ�л�ԭ���Ʊ�������
��д������ϩ���״�Ϊ�л�ԭ���Ʊ�������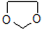 �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��CH3CH2OH
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��CH3CH2OH| Ũ���� |
| 170�� |
| Br2 |
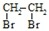
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| �¶ȣ��棩 | 300 | 400 | 500 |
| K�� | 3.1��1015 | 1.66��1014 | 5.3��1013 |
| K�� | 4.0��1020 | 3.6��1018 | 5.7��1017 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ����� | ������Һ�����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.10 | 24.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
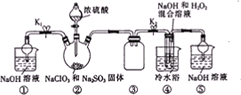 ��֪�������ƣ�NaClO2��Ϊ��ɫ��ĩ��������ˮ��NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2
��֪�������ƣ�NaClO2��Ϊ��ɫ��ĩ��������ˮ��NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com