��������1�������ж�����ԭ���γɵĦļ���Ŀ��Ȼ���жϹ¶Ե�����Ŀ���Դ��ж��ӻ����ͣ���ϼ۲���ӶԻ���ģ�Ϳ��жϷ��ӵĿռ乹�ͣ�
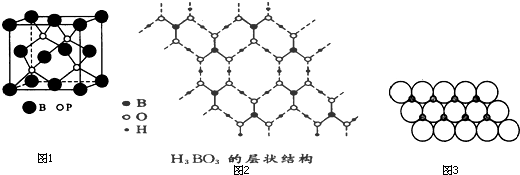
��2����������ľ���ṹ���
��3�������þ�̯�����㺬1molH
3BO
3�ľ����е������
�ڸ���[B��OH��
4]
-������������жϻ�ѧ�������ͣ�
��4��������ͬ������������������ʵĵ��룬��������������ȣ�����һ��N��O����λ������Ϊ�����λ����ʹ��N�Ը�ǿ�������ԣ����ǻ���H-O���ĵ����Ƶ������������Ը���H�����������ӵ���ʽ��ȥ��
��5�������Ȼ��ƵĽṹ֪�������Ӻ����ڵ�������֮��ľ���Ϊ
a��������������������Ӻ˼�ľ����Ǿ�������������Ӻ������Ӿ����
��������ͼƬ֪��ÿ����������ռ�����=1.40��10
-10m��1.40��10
-10m��sin60�㣬ÿ��������������=
g��ÿ������������������ÿƽ�����е���������������ÿƽ�����е�������������
���
�⣺��1��BF
3��Bԭ���γ�3���ļ����¶Ե�����Ϊ
=0����BF
3��Bԭ�ӵļ۲���ӶԸ���=3+0=3���Ҳ����µ��Ӷԣ����Կռ乹����ƽ�������Σ�����ԭ������sp
2�ӻ���
�ʴ�Ϊ��ƽ�������Σ�sp
2��
��2����ͼ��֪��Bԭ���ڶ����Ϻ������ϣ���Bԭ����Ϊ
��8+
��6=4��4��Pԭ���ھ����ڲ�ȫ�����ڸþ�����������Ļ�ѧʽΪBP��������ڵ�������Ϊ���ۼ������������Թ��ۼ��γɵĿռ���״�ṹ�ľ�������ԭ�Ӿ��壻
�ʴ�Ϊ��BP��ԭ�Ӿ��壻
��3����Bԭ���������3�����ӣ�ÿ��Bԭ���γ�3�����ۼ����������������B�������6�����ӣ�һ��H
3BO
3���Ӷ�Ӧ��6�������һ�������Ӧ��2��H
3BO
3���ӣ���˺���1 molH
3BO
3���ӵľ�������3mol�����
�ʴ�Ϊ��6��3��
����������ˮ�����������һˮ������B��OH��
3?H
2O����������������[B��OH��
4]
-��H
+���ӣ��������ܵ����һ����ԭ�ӣ�������������һԪ�[B��OH��
4]
-���еĻ�ѧ������Ϊ���ۼ�����λ����
�ʴ�Ϊ��һ�����ۼ�����λ����
��4���������ֲܷ����룬��һ��������������������˵ڶ������룬����H
3PO
4��K
1Զ����K
2��
�ʴ�Ϊ����һ������������������Ƶڶ����ĵ��룻
��������N��+5�ۣ�N-O-H��O�ĵ��Ӹ���Nƫ�ƣ�������Խ����������ӣ������Խϴ�
�ʴ�Ϊ��������N����5�ۣ�N-O-H��O�ĵ��Ӹ���Nƫ�ƣ�������������ӣ�
��5�������Ȼ��ƵĽṹ֪�������Ӻ����ڵ�������֮��ľ���Ϊ
a��������������������Ӻ˼�ľ����Ǿ�������������Ӻ������Ӿ����
���������������
acm������ͼƬ֪��ÿ����������ռ�����=1.40��10
-10m��1.40��10
-10m��sin60�㣬
��ÿƽ�����е�����������=
| 1 |
| 1.40��10-10m��1.40��10-10m��sin60�� |
��
ÿ��������������=
g������ÿƽ�����е�����������=
��
| 1 |
| 1.40��10-10m��1.40��10-10m��sin60�� |
=1.83��10
-3g��
�ʴ�Ϊ��
a��1.83��10
-3��



 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
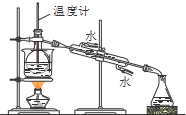
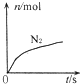 ij���ӷ�Ӧ���漰H2O��ClO-��NH4+��H+��N2��Cl-������������N2�����ʵ�����ʱ��仯��������ͼ��ʾ�������ж���ȷ���ǣ�������
ij���ӷ�Ӧ���漰H2O��ClO-��NH4+��H+��N2��Cl-������������N2�����ʵ�����ʱ��仯��������ͼ��ʾ�������ж���ȷ���ǣ�������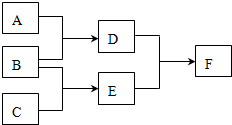 ���� D��E��F�������ʣ�����A��B��C���ɶ�����Ԫ����ɵij������ʣ�D���Ӻ���10�����ӣ�F��һ�ֲ�������Ԫ�ص��Σ�һ��������ת����ϵ���£���ش�
���� D��E��F�������ʣ�����A��B��C���ɶ�����Ԫ����ɵij������ʣ�D���Ӻ���10�����ӣ�F��һ�ֲ�������Ԫ�ص��Σ�һ��������ת����ϵ���£���ش�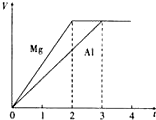 þ�����ֱ����Ũ�ȡ�������Ĺ���ϡ���ᷴӦ����������������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ�����ģ�������
þ�����ֱ����Ũ�ȡ�������Ĺ���ϡ���ᷴӦ����������������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ�����ģ�������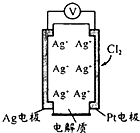 ������ͼ��ʾԭ��ؿɲ���������Cl2���������е������Ag+���������ƶ��Ĺ���
������ͼ��ʾԭ��ؿɲ���������Cl2���������е������Ag+���������ƶ��Ĺ���