
��08�Ͳ���ģ�⣩��18�֣�ijѧϰ��ȤС��̽���ϸɵ�أ�пͲ������̿�ۡ�MnO2��NH4Cl�ȵĺ�״��Ļ������ã����û��յ����ʽ�������ʵ�顣
I����1���ӷϸɵ������ȡNH4Cl��
�� ���øú�״����ȡNH4Clǰ�����IJ���Ϊ��a���ܽ� b�� ��
�� ��ͬѧ���룺���������NH4Cl��Һ�������ᾧ�����գ��Ϳ����Ƶô�����NH4Cl����Լ�ͬѧ�ķ����������۲�˵�����ɣ�______________________________________
��
��2����ȡ������
�� ��ͬѧҪ�Ʊ����ռ��������İ��������и���Ӧ�������к�������
a�����Ȼ�粒�����ȷֽ� b����Ũ��ˮ�����������ƹ�����
c�����������ƹ������Ũ��ˮ�� d�����Ȼ��Ũ��Һ�����������ƹ�����

�� ��ͬѧ��Ϊ������ƿ���������ϣ���ͼ��ʾ�����Ϳ����ռ��������İ�����
������Ϊ���У���˵���������_____________________________��
������Ϊ�����У���˵�������ɣ�___________________________��
��ͬѧ��������õ��İ�����ȡ��ˮ������������̽��ʵ�飺
��3��Ϊ̽��NH3?H2O�Ƿ���������ʡ�
����ͬѧ��Ʒ������£��� ��1.12L�������NH3��ȫ����ˮ�������Һ500mL��
�� ���۷������ݣ���
�ɵó����ۡ�
������ʵ�鲽��ڣ���д������Ŀո��С�
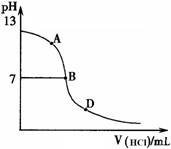 ��4��̽����ˮ������ķ�Ӧ���̡�
��4��̽����ˮ������ķ�Ӧ���̡�
��ͬѧ����������ʵ�飺��25mL������ˮ����
�εμ�ͬŨ�ȵ����ᣬ�ⶨ��Ӧ��������ҺpH����
����pH�仯���ߣ���ͼ������ش�
�����ǡ����ȫ�к�ʱ��pH��Ӧ��_________
����A��B��D������ʱ��Һ��c(NH3?H2O)��c(NH4+)��______mol?L��1����Һ��
�����ӵ�Ũ���ɴ�С��˳��Ϊ ��
III�������̽��
��5�����������̽��ʵ�鱨�档
��̽�����⡿�Ƚ���25�桢0.1mol?L-1��NH3?H2O��Һ��0.1mol?L-1��NH4Cl��Һ�У�NH3?H2O�ĵ���̶���NH4+ˮ��̶ȵ���Դ�С��
��̽���������𰸣�
��1�� | 13 | ���� | 1 |
14 | �˷��������У�ԭ����������ʱ�Ȼ�炙�ֽ� | 1 | |
��2�� | 15 | b d | 2 |
16 | ��a����ͨ�백�� | 1 | |
��3�� | 17 | �ⰱˮ��pH | 2 |
��4�� | 18 | D | 2 |
19 | 0.05 | 2 | |
20 | c(Cl��)��c(NH4��)��c(H��)��c(OH��) | 2 | |
��5�� | 21 | ����1��ȡ25��ĵ������Ũ�Ⱦ�Ϊ0.2mol?L-1�İ�ˮ���Ȼ����Һ��ϲ�������Һ��pH�� ��pH��7����˵��NH3?H2O�ĵ���̶ȴ���NH4+��ˮ��̶ȣ� ��pH��7����˵��NH3?H2O�ĵ���̶ȵ���NH4+��ˮ��̶ȣ� ��pH��7����˵��NH3?H2O�ĵ���̶�С��NH4+ˮ��̶ȣ� | 2 |
3 | |||
21 | ����2���ֱ�ȡ25��Ũ�Ⱦ�Ϊ0.1mol?L-1�İ�ˮ���Ȼ����Һ������pH�ֱ��Ϊa��b�� ��a��14��b����˵��NH3?H2O�ĵ���̶ȴ���NH4+ˮ��̶ȣ� ��a��14��b����˵��NH3?H2O�ĵ���̶ȵ���NH4+ˮ��̶ȣ� ��a��14��b����˵��NH3?H2O�ĵ���̶�С��NH4+ˮ��̶ȡ� | 2 | |
3 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�Ͳ���ģ�⣩ij�¶��£���ͬpH��������������Һ��ˮ���������c(H+)�ֱ���1.0��10��a mol?L-1��1.0��10��bmol?L��1���ڴ��¶��£�������˵����ȷ���ǣ���
A��a��b B��a��b
C��ˮ�����ӻ�Ϊ1.0��10��(7+a) D��ˮ�����ӻ�Ϊ1.0��10��(b+a)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com