
 ČĖĪøĖį£ØÖ÷ŅŖ³É·ÖŹĒŃĪĖį£©¹ż¶ą£¬»įŅżĘšŗܶąĪø²”£®ĻĀĶ¼ĪŖijæ¹ĖįŅ©°ü×°±źĒ©ÉĻµÄ²æ·ÖĪÄ×Ö£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®
ČĖĪøĖį£ØÖ÷ŅŖ³É·ÖŹĒŃĪĖį£©¹ż¶ą£¬»įŅżĘšŗܶąĪø²”£®ĻĀĶ¼ĪŖijæ¹ĖįŅ©°ü×°±źĒ©ÉĻµÄ²æ·ÖĪÄ×Ö£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®·ÖĪö £Ø1£©ÓūÕżČ·½ā“š±¾Ģā£¬Šč“ÓČĖĢåĻū»ÆĻµĶ³µÄĢŲµćŗĶÓ°Ļģ»Æѧ·“Ó¦ĖŁĀŹµÄŅņĖŲæ¼ĀĒ£»
£Ø2£©ĪøĖįµÄÖ÷ŅŖ³É·ÖŹĒŃĪĖį£¬æÉŅŌŗĶAl£ØOH£©3·“Ó¦£¬Š“³ö·“Ó¦·½³ĢŹ½¼“æÉ£»øł¾Ż»Æѧ·“Ó¦·½³ĢŹ½µĆ³öø÷ĪļÖŹÖ®¼äµÄÖŹĮæ±Č£¬ĮŠ³ö±ČĄżŹ½£¬¼“æÉĒó³öAl£ØOH£©3µÄÖŹĮ棬ŌŁÓė±źĒ©ÉĻµÄŗ¬Įæ±Č½Ļ£¬¾ĶæÉÅŠ¶ĻøĆŅ©Ę¬ÖŠĒāŃõ»ÆĀĮµÄŗ¬ĮæŹĒ·ń“ļµ½±ź×¢£®Č»ŗóøł¾ŻÖŹĮæ·ÖŹż¹«Ź½¾ĶæɼĘĖć³öøĆŅ©Ę¬ÖŠĒāŃõ»ÆĀĮµÄÖŹĮæ·ÖŹż£®
½ā“š ½ā£ŗ£Ø1£©Ōö“óŅ»¶ØĮæ¹ĢĢåµÄ±ķĆ껿£ØČē·ŪĖ飩£¬æÉŌö“ó·“Ó¦ĖŁĀŹ£®¾×½ĄŗóĀĮĢ¼ĖįĆ¾Ę¬³ŹŠ”æÅĮ£×“£¬æÉŅŌŌö“óÓėĪøĖįµÄ½Ó“„Ćę£¬Ņņ¶ųŅ×ÓŚ±»Ļū»ÆĪüŹÕĢįøßŅ©Š§£¬
¹Ź“š°øĪŖ£ŗŅ©ĪļŗĶĪøĖį³ä·Ö½Ó“„£¬ŃøĖŁ·¢»ÓŅ©Š§£»
£Ø2£©¢ŁĪøĖįµÄÖ÷ŅŖ³É·ÖŹĒŃĪĖį£¬æÉŅŌŗĶAl£ØOH£©3·¢ÉśÖŠŗĶ·“Ó¦£¬ÉčĒāŃõ»ÆĀĮµÄÖŹĮæĪŖx²ĪÓė·“Ó¦µÄHClµÄÖŹĮæĪŖ£ŗ1.02g/mL”Į6.0mL”Į5%”Ö0.306g
Al£ØOH£©3+3HCl=AlCl3+3H2O
78 109.5
x 0.306g
½āÖ®µĆ£»x”Ö0.218g
0.218g=218mg£¼250mg
¹ŹøĆŅ©Ę¬ÖŠĒāŃõ»ÆĀĮµÄŗ¬Įæƻӊ“ļµ½±ź×¢£¬
“š£ŗøĆŅ©Ę¬ÖŠĒāŃõ»ÆĀĮµÄŗ¬Įæƻӊ“ļµ½±ź×¢£»
¢ŚøĆŅ©Ę¬ÖŠĒāŃõ»ÆĀĮµÄÖŹĮæ·ÖŹżĪŖ $\frac{0.218g}{0.5g}$”Į100%”Ö43.6%£®
“š£ŗøĆŅ©Ę¬ÖŠĒāŃõ»ÆĀĮµÄÖŹĮæ·ÖŹżŹĒ43.6%£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éѧɜ¶ŌĖį¼ī·¢ÉśÖŠŗĶ·“Ó¦ŗĶÓ°Ļģ»Æѧ·“Ó¦ĖŁĀŹµÄŅņĖŲµÄÖŖŹ¶µćµÄÕĘĪÕĒéæö£¬ŅŌ¼°ŌĖÓĆ»Æѧ·½³ĢŹ½ŗĶÖŹĮæ·ÖŹż¹«Ź½½ųŠŠ¼ĘĖćµÄÄÜĮ¦£®Ń§ÉśŠėČĻÕę·ÖĪöŅŃÖŖĢõ¼ž£¬ÕżČ·ŹéŠ“»Æѧ·½³ĢŹ½£¬²ÅÄÜÕżČ·½ā“š£®


 ÓÅ°Ł·ÖæĪŹ±»„¶ÆĻµĮŠ“š°ø
ÓÅ°Ł·ÖæĪŹ±»„¶ÆĻµĮŠ“š°ø æŖŠÄĶÜדŌŖ×÷ŅµĻµĮŠ“š°ø
æŖŠÄĶÜדŌŖ×÷ŅµĻµĮŠ“š°ø æĪŹ±ÕĘæŲĖęĢĆĮ·Ļ°ĻµĮŠ“š°ø
æĪŹ±ÕĘæŲĖęĢĆĮ·Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| ±ąŗÅ | ¢Ł | ¢Ś | ¢Ū | ¢Ü |
| ×°ÖĆ |  |  |  |  |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ńõ»ÆŠŌ£ŗClO-£¾I2£¾SO42- | |
| B£® | Ą¶É«ĻūŹ§µÄŌŅņŹĒNa2SO3ČÜŅŗ·“Ӧɜ³ÉSO2¾ßÓŠĘư׊Ō | |
| C£® | µķ·ŪKIČÜŅŗ±äĄ¶ŹĒŅņĪŖI-±»ClO-Ńõ»ÆĪŖI2£¬I2Óöµķ·Ū±äĄ¶ | |
| D£® | Čō½«Na2SO3ČÜŅŗ¼ÓČėäåĖ®£¬äåĖ®ĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
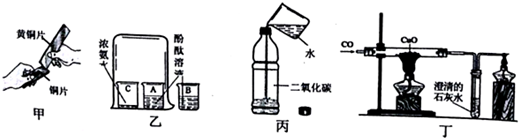
| A£® | ¼×ŹµŃéÖŠ»ĘĶʬÄÜŌŚĶʬÉĻæĢ»³öŗŪ¼£æÉŅŌĖµĆ÷»ĘĶµÄÓ²¶Č±ČĶʬ“ó | |
| B£® | ŅŅŹµŃé¼ČæÉŅŌĖµĆ÷·Ö×ÓŌŚ²»Ķ£µÄŌĖ¶Æ×Å£¬ÓÖæÉŅŌĖµĆ÷°±Ė®ĻŌ¼īŠŌ | |
| C£® | ±ūŹµŃé¼ČæÉŅŌĖµĆ÷¶žŃõ»ÆĢ¼Ņ×ČÜÓŚĖ®£¬ÓÖæÉŅŌĖµĆ÷¶žŃõ»ÆĢ¼¾ßÓŠĖįŠŌ | |
| D£® | ¶”ŹµŃé¼ČæÉŅŌĖµĆ÷Ņ»Ńõ»ÆĢ¼¾ßÓŠ»¹ŌŠŌ£¬ÓÖæÉŅŌĖµĆ÷Ņ»Ńõ»ÆĢ¼¾ßÓŠæÉČ¼ŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
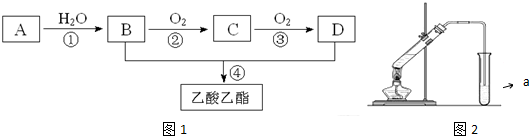
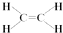 £¬CµÄ½į¹¹¼ņŹ½£ŗCH3CHO£®
£¬CµÄ½į¹¹¼ņŹ½£ŗCH3CHO£®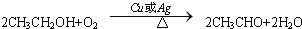 £»·“Ó¦ĄąŠĶ£ŗŃõ»Æ·“Ó¦£®
£»·“Ó¦ĄąŠĶ£ŗŃõ»Æ·“Ó¦£®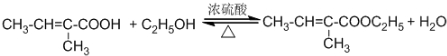 £¬ĪŖ“ÓøĆŹµŃéŗóµÄ»ģŗĻĘųĢåÖŠ·ÖĄė³öŅŅĖįŅŅõ„£¬ÓŅ²ąŹŌ¹ÜÖŠĖłŃ”ÓƵďŌ¼ĮaŹĒ±„ŗĶĢ¼ĖįÄĘČÜŅŗ£¬aŹŌ¼ĮµÄ×÷ÓĆŹĒÖŠŗĶŅŅĖį”¢ĪüŹÕŅŅ“¼”¢½µµĶŅŅĖįŅŅõ„ŌŚČÜŅŗÖŠµÄČܽā¶Č£¬ÓŠĄūÓŚ·Ö²ćĪö³ö£®
£¬ĪŖ“ÓøĆŹµŃéŗóµÄ»ģŗĻĘųĢåÖŠ·ÖĄė³öŅŅĖįŅŅõ„£¬ÓŅ²ąŹŌ¹ÜÖŠĖłŃ”ÓƵďŌ¼ĮaŹĒ±„ŗĶĢ¼ĖįÄĘČÜŅŗ£¬aŹŌ¼ĮµÄ×÷ÓĆŹĒÖŠŗĶŅŅĖį”¢ĪüŹÕŅŅ“¼”¢½µµĶŅŅĖįŅŅõ„ŌŚČÜŅŗÖŠµÄČܽā¶Č£¬ÓŠĄūÓŚ·Ö²ćĪö³ö£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
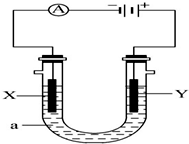 µē½āŌĄķŌŚ»Æѧ¹¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ£®ČēĶ¼±ķŹ¾Ņ»øöµē½ā³Ų£¬X”¢Y¶¼ŹĒ¶čŠŌµē¼«£¬aŹĒ±„ŗĶNaClČÜŅŗ£¬ŹµŃéæŖŹ¼Ź±£¬Ķ¬Ź±ŌŚĮ½±ßø÷µĪČė¼øµĪ·ÓĢŖČÜŅŗ£¬Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ
µē½āŌĄķŌŚ»Æѧ¹¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ£®ČēĶ¼±ķŹ¾Ņ»øöµē½ā³Ų£¬X”¢Y¶¼ŹĒ¶čŠŌµē¼«£¬aŹĒ±„ŗĶNaClČÜŅŗ£¬ŹµŃéæŖŹ¼Ź±£¬Ķ¬Ź±ŌŚĮ½±ßø÷µĪČė¼øµĪ·ÓĢŖČÜŅŗ£¬Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
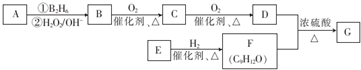
 £»øĆ·“Ó¦µÄ·“Ó¦ĄąŠĶĪŖČ”“ś·“Ó¦»ņõ„»Æ·“Ó¦
£»øĆ·“Ó¦µÄ·“Ó¦ĄąŠĶĪŖČ”“ś·“Ó¦»ņõ„»Æ·“Ó¦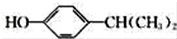 £®
£® ĪŖŌĮĻŅ²æÉŗĻ³ÉF£¬Ēė²Īæ¼ĢāÄæÖŠµÄĻą¹ŲŠÅĻ¢Š“³öĻąÓ¦µÄŗĻ³ÉĀ·ĻßĶ¼£Ø·“Ó¦Ģõ¼žÖŠµÄŹŌ¼ĮŠ“ŌŚ¼żĶ·ÉĻ·½£¬ĘäĖūŠ“ŌŚ¼żĶ·ĻĀ·½£©£ŗ
ĪŖŌĮĻŅ²æÉŗĻ³ÉF£¬Ēė²Īæ¼ĢāÄæÖŠµÄĻą¹ŲŠÅĻ¢Š“³öĻąÓ¦µÄŗĻ³ÉĀ·ĻßĶ¼£Ø·“Ó¦Ģõ¼žÖŠµÄŹŌ¼ĮŠ“ŌŚ¼żĶ·ÉĻ·½£¬ĘäĖūŠ“ŌŚ¼żĶ·ĻĀ·½£©£ŗ £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Ō×Ó¼°ĘäĄė×ÓµÄŗĖĶāµē×Ó²ćŹżµČÓŚøĆŌŖĖŲĖłŌŚµÄÖÜĘŚŹż | |
| B£® | ŌŖĖŲÖÜĘŚ±ķÖŠ“ÓIIIB×åµ½IIB×å 10øöׯŠŠµÄŌŖĖŲ¶¼ŹĒ½šŹōŌŖĖŲ | |
| C£® | ³żŗ¤ĶāµÄĻ”ÓŠĘųĢåŌ×ÓµÄ×īĶā²ćµē×ÓŹż¶¼ŹĒ8 | |
| D£® | Ķ¬Ņ»ŌŖĖŲµÄø÷ÖÖĶ¬Ī»ĖŲµÄĪļĄķŠŌÖŹ”¢»ÆѧŠŌÖŹ¾łĻąĶ¬ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com