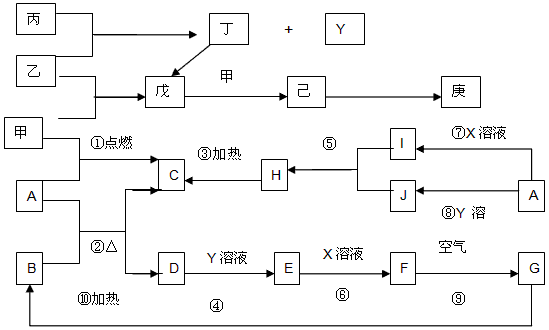
����A~J����~����Щ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ���е�ת����ʡ���˲������������֪��A��D��Ϊ�������ʣ�A��C��H��I��J����ͬһ��Ԫ�أ�B��D��E��F��G����ͬһ��Ԫ�أ�������X��Y�������ȼҵ�Ƶã�X��Y��Ͽ�����ʳ�Ρ��ס�����Ϊ�����µ���̬�ǽ������ʣ��ұ���һ�ֻ���ɫ�ж����ʣ�����Y�����а������ɡ�
��ش�Щ�����⣺
��1������X�ĵ���ʽ____________��д���Ļ�ѧʽ____________��
��2��д����Ӧ�ߵ����ӷ���ʽ________________________����Ӧ��Ļ�ѧ����ʽ________________________��
��3��д��A��B�ڸ���ʱ��Ӧ�Ļ�ѧ����ʽ��������ӵ�ת����Ŀ�ͷ���________________________
��4����������~��ķ�Ӧ�У����ڷ�������ԭ��Ӧ����____________����д �� ~���е���ţ���
��5���ҷ����л�ѧ����������____________������Ӽ����Լ������ҷ��ӵĿռ乹����
____________��
��6������ʱ����D�������Ũ��Һ�в�������Ӧ����ԭ����________________________�����ڼ���ʱ��D����������Ũ��Һ��Ӧ�����ӷ���ʽ��________________________��