
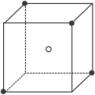
��1���þ���Ļ�ѧʽΪ____________��
��2�������о������������X��Y�γɵļнǡ�XYX�Ƕ�Ϊ______________����ǵĶ�������
��3���˾�����X���Ӹ���=___________���г���ʽ����Y���Ӹ���=__________���г���ʽ����
��4����þ����Ħ������ΪM g��mol-1��������ܶ�Ϊ�� g��cm-3�������ӵ�����ΪNa���������������������X��ľ���Ϊ_____________cm��
 �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
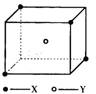
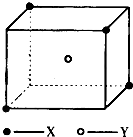 ij���Ӿ��徧���ṹ��ͼ��ʾ��Xλ��������Ķ��㣬Yλ�����������ģ��Է�����
ij���Ӿ��徧���ṹ��ͼ��ʾ��Xλ��������Ķ��㣬Yλ�����������ģ��Է������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
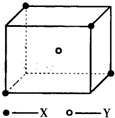 ij���Ӿ��徧���ṹ��ͼ��ʾ��xλ��������Ķ��㣬Yλ�����������ģ��Է�����ÿ��xͬʱ������
ij���Ӿ��徧���ṹ��ͼ��ʾ��xλ��������Ķ��㣬Yλ�����������ģ��Է�����ÿ��xͬʱ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 3 |
| ||
| 2 |
| 3 |
| ||
| 2 |
| ���ӻ����� | H2S | CO32- | PH3 | ClO4-- |
| �ռ乹�� | V�� V�� |
ƽ�������� ƽ�������� |
������ ������ |
�������� �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
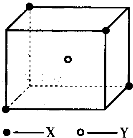 ij���Ӿ��徧���ṹ��ͼ��ʾ��xλ��������Ķ��㣬Yλ�����������ģ��Է�����
ij���Ӿ��徧���ṹ��ͼ��ʾ��xλ��������Ķ��㣬Yλ�����������ģ��Է������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ����10���¿���ѧ�Ծ����°ࣩ�������棩 ���ͣ������
��10�֣�ij���Ӿ��徧���ṹ��ͼ��ʾ��Xλ��������Ķ��㣬Yλ�����������ġ��Է�����
��1��������ÿ��Yͬʱ������____��X���þ���Ļ�ѧʽΪ_____ ��
��2����������ÿ��X��Χ������ӽ��Ҿ�����ȵ�X����________����
��3�������о��������2��X��1��Y�γɵļнǡ�XYX�Ķ���Ϊ_______����4����þ���Ļ�ѧʽ��ʽ��ΪM�������ܶ�Ϊ�ѣ������ӵ�����ΪNA�������������������X���ļ�ľ���Ϊ__________ ��
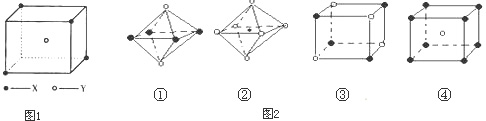
��5������ͼ���Ǵ�NaCl��CsCl����ṹͼ�зָ�����IJ��ֽṹͼ�����ж�NaCl����ṹ��ͼ����
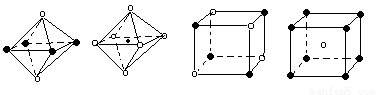
�� �� �� ��
A���� B���� C���� D����
��6���ü۲���ӶԻ������ۣ�VSEPR���ж����з��ӻ����ӵĿռ乹��
|
���ӻ����� |
H2S |
SnCl62- |
PH3 |
ClO4-- |
|
�ռ乹�� |
|
|
|
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com