
����Ԫ������Ԫ��������ռ����Ҫ��λ,�ش������й��������⡣
(1)������������Ϊ����θ�������ڷ�ҩ,��������������������____________�ԡ�
(2)��γ�ȥSiO2�е�Al2O3����(�����ӷ���ʽ��ʾ)____________________��
(3)������ڵ���������ȡ������,����0.2 mol���ӷ���ת��,���������ܵõ�����________g��
(4)��100 mL 0.5 mol��L-1AlCl3������0.5 mol��L-1��NaOH��Һ,���õ���ɫ����2.34�ˡ�����NaOH��Һ�����Ϊ____________��
��������(1)������������θ�ᷴӦ,���������������ļ���;
(2)��ȥSiO2�е�Al2O3����,Ӧѡ������ʹ�������ܽ�,���˳�ȥ;
(3)�������������0.2 mol���ӷ���ת��,���������ʵ���Ϊ0.2/3 mol,����Ϊ1.8 g;
(4)�����������������ʵ���Ϊ2.34 g /78 g��mol-1=0.03 mol,�������������Ʋ���,�������Ƶ����ʵ���Ϊ0.03 mol��3=0.09 mol,���Ϊ180 mL;�������������ƹ���,����ԭ���غ�֪������0.02 molNa[Al(OH)4],�������Ƶ����ʵ���Ϊ0.03 mol��3+0.02 mol��4=0.17 mol,���Ϊ340 mL��
��:(1)�(2)Al2O3+6H+====2Al3++3H2O
(3)1.8��(4)180 mL��340 mL


 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ԭ��Ӧ�����ֻ�����Ӧ���͵Ĺ�ϵ����ͼ��ʾ�������л�ѧ��Ӧ���ڢ��������(����)
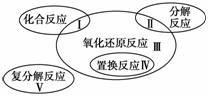
A��Cl2��2KBr===Br2��2KCl
B��2NaHCO3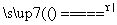 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
C��4Fe(OH)2��O2��2H2O===4Fe(OH)3
D��2Na2O2��2CO2===2Na2CO3��O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ѧʵ��ʱ�����Ǿ���Ҫ�õ������������ѧ֪ʶ�ش��������⣺
(1)�����������������ʣ�
________________________________________________________________________
(2)������Щʵ�����õ�����������Щʵ��������������ʲô��Ӧ����д�����ʵ��Ļ�ѧ����ʽ����ۡ�__________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
(3)������Щ���ʿ��Դ������������Щʵ�飿
________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)CO2����Է�������Ϊ______________������Ħ������Ϊ________��
(2)�ڱ�״���£�0.5 mol�κ�����������ԼΪ________��
(3)4 g H2��22.4 L(��״��)CO2��ȣ�����������Ŀ�϶����________��
(4)0.01 molij���������Ϊ0.28 g���������Ħ������Ϊ__________���ڱ�״���£�������������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȥþ���к��е���������,��ѡ�õ��Լ���(����)
A.���ᡡ B.NaOH��Һ  C.���� D.��ˮ
C.���� D.��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ǯ��סһС������ᴦ�������������ھƾ����ϼ������ۻ��������ۻ������������䡣���й���ʵ������Ľ��Ͳ���ȷ����(����)
A�����ڿ������ܺܿ��γ�����Ĥ B����������Ĥ��ס���ۻ�����
C�����������۵������ D�����������¶ȵͣ�δ�ۻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧС���ڳ����²ⶨһ��������ijͭ���������ͭ���������������������ʵ�鷽����
������ͭ�������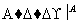 �ⶨ������������
�ⶨ������������
������ͭ�������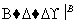 �ⶨʣ����������
�ⶨʣ����������
�����й��ж��в���ȷ����(����)
A����ҺA��B�������������NaOH��Һ
B����ҺA��B������ѡ��ϡ����
C������ҺBѡ��Ũ���ᣬ����ͭ����������ƫС
D��ʵ�鷽���������ʵʩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���������(����)
A����1 L 1 mol��L��1��NaCl��Һ��ȡ��10 mL����Ũ������ 1 mol��L��1
B���Ƴ�0.5 L 10 mol��L��1�����ᣬ��ҪHCl����112 L(��״��)
C��0.5 L 2 mol��L��1��BaCl2��Һ�У�Ba2����Cl������Ϊ3��6.02��1023
D��10 g 98%������(�ܶ�Ϊ1.84 g��cm��3)��10 mL 18.4 mol��L��1�������Ũ���Dz�ͬ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ�������һ�����ʵ���Ũ�ȵ���Һ,���в�����ʹ��õ���ҺŨ��ƫС����(����)
A.����ƿ��ԭ����������ˮ
B.��Һ���ձ�ת�Ƶ�����ƿ��û��ϴ���ձ�
C.δ��ȴ������Һת�Ƶ�����ƿ
D.��ˮδ�ﵽ����ƿ�̶���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com