
ͭ����Ҫ�Ľ������ϡ�
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ���÷�Ӧ��������Ϊ _______ ������ͭ��ȡ��ͭ�����ʱ������������ _______ �����Һ�б��뺬�е��������� _______ ��
(2)��100 mL 18 mol/LŨ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4< __ mol��
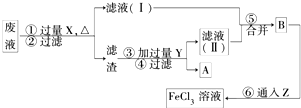
(3)���ӹ�ҵ����30����FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ������н�õ�FeCl3��Һ���������ʵ�����̡�

���������У������Լ��Ļ�ѧʽΪ��X _______ ��Y _______ Z _______ ���ڢ���Ӧ�����ӷ���ʽΪ _______ ��
 ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�챱���б�ʦ��ѧУ�����ڶ����¿���ѧ�Ծ����������� ���ͣ������
ͭ����Ҫ�Ľ������ϣ�
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ����ѧ����ʽΪ ���÷�Ӧ��������Ϊ________ ��
(2)��100 mL 18 mol��L��1Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4��________mol.
(3)���ӹ�ҵ����30%��FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ�������µ�FeCl3��Һ���������ʵ�����̣�
���������У������Լ��Ļ�ѧʽΪ��
X________________��Y____________��Z____________��
�ڢ���Ӧ�����ӷ���ʽΪ___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱���и����ڶ����¿���ѧ�Ծ��������棩 ���ͣ������
ͭ����Ҫ�Ľ������ϣ�
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ����ѧ����ʽΪ ���÷�Ӧ��������Ϊ________ ��
(2)��100 mL 18 mol��L��1 Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4��________mol.
(3)���ӹ�ҵ����30%��FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ�������µ�FeCl3��Һ���������ʵ�����̣�

���������У������Լ��Ļ�ѧʽΪ��
X________________��Y____________��Z____________��
�ڢ���Ӧ�����ӷ���ʽΪ___________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com