
| A�����ٴ���CO32-��Cl-�е�һ�� |
| B��Cl-һ�����ڣ�K+���ܴ��� |
| C��Cl-һ�����ڣ���c��Cl-����0.6mol?L-1 |
| D����Һ�����ٴ���4������ |
| 0.8g |
| 160g/mol |
| 2.33g |
| 233g/mol |
| 0.8g |
| 160g/mol |
| 2.33g |
| 233g/mol |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | Na+ H+ Ba2+ | ||||
| ������ | OH- CO
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
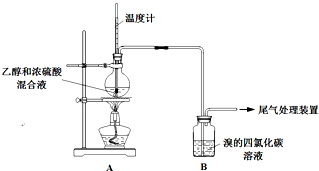 ʵ��������ϩʱ��������������ʹBr2 �����Ȼ�̼��Һ��ɫ���ס���ͬѧ����ͼʵ����֤�����������Ѽ��飬���ּг�װ���ԣ���
ʵ��������ϩʱ��������������ʹBr2 �����Ȼ�̼��Һ��ɫ���ס���ͬѧ����ͼʵ����֤�����������Ѽ��飬���ּг�װ���ԣ���| �� �� | �� �� |
| ��ȼ�ƾ��ƣ� ������170�� | ��A����ƿ��Һ�彥����� ��B����������ð������Һ����ɫ |
| �� | |
| ʵ����ϣ� ��ϴ��ƿ | ��A����ƿ�ڸ���������ɫ����״��д̼�����ζ�ݳ� |
| �� �� | �� �� | |
| �� | ��A��B������һ��װ��ij���Լ���ϴ��ƿ | Br2��CCl4��Һ��ɫ |
�� | ��A���ӵ�װ�����£�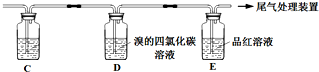 | D����Һ�ɺ���ɫ��Ϊdz����ɫʱ��E����Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ij��Һ������ɫ��Ӧʱ������ɫΪ��ɫ�������Һ��һ����Na+��������K+ |
| B����ij��Һ�м���AgNO3��Һ������ɫ����������ϴ����ʱ�������ܽ⣬��ȷ����Cl-���� |
| C����������ʱ����ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ����CO32-���� |
| D���ֱ���Mg2+��Cu2+��Fe2+��Na+�������ӵ���Һ��ֻ��NaOH��Һ�����ܼ���ɹ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ܢڢ٢ڢ� | B���٢ڢۢ� |
| C���ܢ٢ڢ� | D���ڢ٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
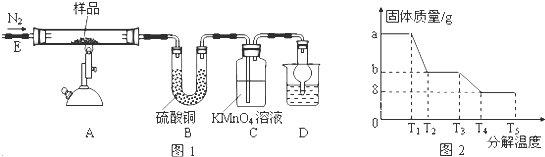
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
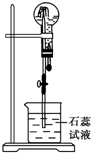 ����A��һ�ְ�ɫ���壬����ŨNaOH��Һ���ȣ��ų���ɫ����B����Բ����ƿ�ռ������B������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��A��Ũ���ᷴӦ���ų���ɫ����C����Բ����ƿ�ռ������C������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��
����A��һ�ְ�ɫ���壬����ŨNaOH��Һ���ȣ��ų���ɫ����B����Բ����ƿ�ռ������B������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��A��Ũ���ᷴӦ���ų���ɫ����C����Բ����ƿ�ռ������C������ͼ��ʾװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com