
 »ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ£ØČēĶ¼£©£¬ÓĆ»·¼ŗ“¼Öʱø»·¼ŗĻ©£®
»ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ£ØČēĶ¼£©£¬ÓĆ»·¼ŗ“¼Öʱø»·¼ŗĻ©£®
| ĆÜ¶Č£Øg/cm3£© | ČŪµć£Ø”ę£© | ·Šµć£Ø”ę£© | ČܽāŠŌ | |
| »·¼ŗ“¼ | 0.96 | 25 | 161 | ÄŃČÜÓŚĖ® |
| »·¼ŗĻ© | 0.81 | -103 | 83 | ÄŃČÜÓŚĖ® |
·ÖĪö £Ø1£©¢Łøł¾ŻÖĘŅŅĻ©ŹµŃéµÄÖŖŹ¶£¬·¢Éś×°ÖĆAÖŠĖé“ÉʬµÄ×÷ÓĆŹĒ·ĄÖ¹±©·Š£¬ÓÉÓŚÉś³ÉµÄ»·¼ŗĻ©µÄ·ŠµćĪŖ83”ę£¬ŅŖµĆµ½ŅŗĢ¬»·¼ŗĻ©£¬µ¼¹ÜB³żĮĖµ¼ĘųĶā»¹¾ßÓŠĄäÄż×÷ÓĆ£¬±ćÓŚ»·¼ŗĻ©ĄäÄż£»
¢Ś±łĖ®Ō”µÄÄæµÄŹĒ½µµĶ»·¼ŗĻ©ÕōĘųµÄĪĀ¶Č£¬Ź¹ĘäŅŗ»Æ£»
£Ø2£©¢Ł»·¼ŗĻ©²»ČÜÓŚĀČ»ÆÄĘČÜŅŗ£¬ĒŅĆܶȱČĖ®Š”£¬·Ö²ćŗó»·¼ŗĻ©ŌŚÉĻ²ć£¬ÓÉÓŚ·ÖŅŗŗó»·¼ŗĻ©“ÖĘ·ÖŠ»¹ŗ¬ÓŠÉŁĮæµÄĖįŗĶ»·¼ŗ“¼£¬Ģį“æ²śĪļŹ±ÓĆc£ØNa2CO3ČÜŅŗ£©Ļ“µÓæɳżČ„Ėį£»
¢Śøł¾Ż±ķÖŠŹż¾ŻæÉÖŖ£¬Įó·Ö»·¼ŗĻ©µÄ·ŠµćĪŖ83”ę£»
A”¢ČōĢįĒ°ŹÕ¼Æ£¬²śĘ·ÖŠ»ģÓŠŌÓÖŹ£¬Źµ¼Ź²śĮæøßÓŚĄķĀŪ²śĮ棻
B”¢ÖĘČ”µÄ»·¼ŗĻ©ĪļÖŹµÄĮæŌö“ó£¬ŹµŃéÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæøßÓŚĄķĀŪ²śĮ棻
C”¢“Ö²śĘ·ÖŠ»ģÓŠ»·¼ŗ“¼£¬µ¼ÖĀ²ā¶ØĻūŗĵĻ·¼ŗ“¼ĮæŌö“ó£¬ÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæµĶÓŚĄķĀŪ²śĮ棻
½ā“š ½ā£ŗ£Ø1£©¢Łøł¾ŻÖĘŅŅĻ©ŹµŃéµÄÖŖŹ¶£¬·¢Éś×°ÖĆAÖŠĖé“ÉʬµÄ×÷ÓĆŹĒ·ĄÖ¹±©·Š£¬ÓÉÓŚÉś³ÉµÄ»·¼ŗĻ©µÄ·ŠµćĪŖ83”ę£¬ŅŖµĆµ½ŅŗĢ¬»·¼ŗĻ©£¬µ¼¹ÜB³żĮĖµ¼ĘųĶā»¹¾ßÓŠĄäÄż×÷ÓĆ£¬±ćÓŚ»·¼ŗĻ©ĄäÄż£®
¹Ź“š°øĪŖ£ŗĄäÄż£»
¢Ś±łĖ®Ō”µÄÄæµÄŹĒ½µµĶ»·¼ŗĻ©ÕōĘųµÄĪĀ¶Č£¬Ź¹ĘäŅŗ»Æ£¬
¹Ź“š°øĪŖ£ŗŹ¹»·¼ŗĻ©Ņŗ»Æ£¬¼õÉŁ»Ó·¢£»
£Ø4£©¢Ł»·¼ŗĻ©ŹĒĢžĄą£¬²»ČÜÓŚĀČ»ÆÄĘČÜŅŗ£¬ĒŅĆܶȱČĖ®Š”£¬Õńµ“”¢¾²ÖĆ”¢·Ö²ćŗó»·¼ŗĻ©ŌŚÉĻ²ć£¬ÓÉÓŚ·ÖŅŗŗó»·¼ŗĻ©“ÖĘ·ÖŠ»¹ŗ¬ÓŠÉŁĮæµÄĖįŗĶ»·¼ŗ“¼£¬ĮŖĻė£ŗÖʱøŅŅĖįŅŅõ„Ģį“æ²śĪļŹ±ÓĆC£ØNa2CO3ČÜŅŗ£©Ļ“µÓæɳżČ„Ėį£»
¹Ź“š°øĪŖ£ŗÉĻ£»C£»
¢Śøł¾Ż±ķÖŠŹż¾ŻæÉÖŖ£¬Įó·Ö»·¼ŗĻ©µÄ·ŠµćĪŖ83”ę£¬¹ŹŹÕ¼Æ²śĘ·Ó¦æŲÖĘĪĀ¶ČŌŚ83”ę×óÓŅ£»
A”¢ÕōĮ󏱓Ó70”ęæŖŹ¼ŹÕ¼Æ²śĘ·£¬ĢįĒ°ŹÕ¼Æ£¬²śĘ·ÖŠ»ģÓŠŌÓÖŹ£¬Źµ¼Ź²śĮæøßÓŚĄķĀŪ²śĮ棬¹ŹA“ķĪó£»
B”¢»·¼ŗ“¼Źµ¼ŹÓĆĮæ¶ąĮĖ£¬ÖĘČ”µÄ»·¼ŗĻ©µÄĪļÖŹµÄĮæŌö“ó£¬ŹµŃéÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæøßÓŚĄķĀŪ²śĮ棬¹ŹB“ķĪó£»
C”¢Čō“Ö²śĘ·ÖŠ»ģÓŠ»·¼ŗ“¼£¬µ¼ÖĀ²ā¶ØĻūŗĵĻ·¼ŗ“¼ĮæŌö“ó£¬ÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæµĶÓŚĄķĀŪ²śĮ棬¹ŹCÕżČ·£»£¬¹ŹŃ”C£»
¹Ź“š°øĪŖ£ŗ83”ę£»C£»
µćĘĄ ±¾Ģāæ¼²éĮĖŅŅĖįŅŅõ„µÄÖʱø£¬ŅŌ»·¼ŗ“¼Öʱø»·¼ŗĻ©µÄŹµŃé·½·Ø£¬×ŪŗĻæ¼²éĮĖĪļÖŹµÄ·ÖĄė·½·Ø£¬ĄäÄż£¬²śĮæµÄ·ÖĪöµČ£¬ÄѶȏŹÖŠ£¬æ¼²éѧɜ½ā¾öŹµ¼ŹĪŹĢāµÄÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 14C£ŗ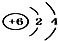 | B£® | 16O£ŗ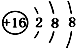 | C£® | Li+£ŗ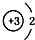 | D£® | H-£ŗ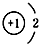 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
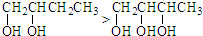
| A£® | ¢Ł¢Ü | B£® | ¢Ś¢Ü¢Ż | C£® | ¢Ł¢Ś¢Ū | D£® | ¢Ū¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ńõ»ÆĪļ£ŗCuO”¢NO”¢SO2”¢H2O | |
| B£® | ¼ī£ŗNaOH”¢KOH”¢Ba£ØOH£©2”¢Na2CO3 | |
| C£® | ¼īŠŌŃõ»ÆĪļ£ŗNa2O”¢CaO”¢Al2O3”¢Na2O2 | |
| D£® | µē½āÖŹ£ŗKNO3”¢Cl2”¢HCl”¢BaSO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅĄ¾Ż¶”“ļ¶ūĻÖĻóæɽ«·ÖÉ¢Ļµ»®·ÖĪŖČÜŅŗ”¢½ŗĢåÓė×ĒŅŗ | |
| B£® | PM2.5£ØĪ¢Į£Ö±¾¶Ō¼ĪŖ2.5”Į10-6 m£©·ÖÉ¢ŌŚæÕĘųÖŠŠĪ³ÉĘųČܽŗ | |
| C£® | ¹āµ¼ĻĖĪ¬”¢¾ŪŅŅĻ©”¢ÓĶÖ¬¶¼ŹĒøß·Ö×Ó»ÆŗĻĪļ | |
| D£® | “óĪķµÄŠĪ³ÉÓėĘū³µµÄĪ²ĘųÅÅ·ÅÓŠŗÜ“ó¹ŲĻµ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
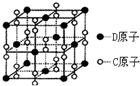 ŅŃÖŖA”¢B”¢C”¢D”¢E¶¼ŹĒŌŖĖŲÖÜĘŚ±ķÖŠµÄĒ°36ŗÅŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£®BŌ×Ó»łĢ¬Ź±PŌ×Ó¹ģµĄÉĻÓŠ3øöĪ“³É¶Ōµē×Ó£¬Ęä×īøßÕż¼ŪÓė×īµĶøŗ¼Ū“śŹżŗĶĪŖ2£¬CµÄ¼Ū²ćµē×ÓÅŲ¼Ź½ĪŖns2npn+2£¬ĘäĒā»ÆĪļŹĒĶ¬×åŌŖĖŲĖłŠĪ³ÉµÄĒā»ÆĪļÖŠ·Šµć×ī“ó£®»ÆŗĻĪļAC2ĪŖ·Ē¼«ŠŌ·Ö×Ó£¬DŌŖĖŲµÄŌ×ÓŗĖĶā¹²ÓŠ20ÖÖ²»Ķ¬ŌĖ¶ÆדĢ¬µÄµē×Ó£®EŹĒŌŖĖŲÖÜĘŚ±ķµŚĖÄÖÜĘŚµŚ9ĮŠŌŖĖŲ£®Ēė»Ų“š£ŗ
ŅŃÖŖA”¢B”¢C”¢D”¢E¶¼ŹĒŌŖĖŲÖÜĘŚ±ķÖŠµÄĒ°36ŗÅŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£®BŌ×Ó»łĢ¬Ź±PŌ×Ó¹ģµĄÉĻÓŠ3øöĪ“³É¶Ōµē×Ó£¬Ęä×īøßÕż¼ŪÓė×īµĶøŗ¼Ū“śŹżŗĶĪŖ2£¬CµÄ¼Ū²ćµē×ÓÅŲ¼Ź½ĪŖns2npn+2£¬ĘäĒā»ÆĪļŹĒĶ¬×åŌŖĖŲĖłŠĪ³ÉµÄĒā»ÆĪļÖŠ·Šµć×ī“ó£®»ÆŗĻĪļAC2ĪŖ·Ē¼«ŠŌ·Ö×Ó£¬DŌŖĖŲµÄŌ×ÓŗĖĶā¹²ÓŠ20ÖÖ²»Ķ¬ŌĖ¶ÆדĢ¬µÄµē×Ó£®EŹĒŌŖĖŲÖÜĘŚ±ķµŚĖÄÖÜĘŚµŚ9ĮŠŌŖĖŲ£®Ēė»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆ“ż²āŅŗČóĻ“µĪ¶ØÓƵÄ׶ŠĪĘæ | |
| B£® | ³£ĪĀĻĀ£¬Ä³Ķ¬Ń§ÓĆpHŹŌÖ½²āµĆKClČÜŅŗµÄpHŌ¼ĪŖ7.0 | |
| C£® | ÅäÖĘFe2£ØSO4£©3ČÜŅŗŹ±£¬ĻČ°ŃFe2£ØSO4£©3¾§ĢåČÜÓŚÅØĮņĖįŗóĻ”ŹĶ | |
| D£® | ÓĆ¼īŹ½µĪ¶Ø¹Ü×¼Č·ŅĘČ”KMnO4ČÜŅŗ£¬Ģå»żĪŖ21.50mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ņ»¶ØĪĀ¶ČŗĶŃ¹ĒæĻĀ£¬ĘųĢ¬ĪļÖŹµÄĢå»żÖ÷ŅŖÓɹ¹³ÉĘųĢåµÄ·Ö×ӵē󊔾ö¶Ø | |
| B£® | “Ó1 L 2 mol/LµÄH2SO4ČÜŅŗÖŠČ”³ö0.5 L£¬øĆČÜŅŗÖŠĒāĄė×ÓµÄÅضČĪŖ4mol/L | |
| C£® | Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬30mLA2ĘųĢåŗĶ10mL B2ĘųĢåĒ”ŗĆĶźČ«·“Ӧɜ³É20mLCĘųĢ壬ŌņC»ÆѧŹ½ĪŖ A3 B»ņB A3 | |
| D£® | Ķ¬ĪĀĶ¬Ń¹ĻĀČĪŗĪĘųĢåµÄ·Ö×Ó¼ä¾ąĄė¼øŗõĻąµČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2O×÷ÅäĢåŹ±£¬CuÓėH2OŠĪ³É¦Ņ¼ü | B£® | H2OµÄÅäĪ»ÄÜĮ¦“óÓŚNH3 | ||
| C£® | ŅŅ“¼æɼõŠ”Ąė×Ó¾§ĢåµÄČܽā¶Č | D£® | Cu£ØNH3£©4SO4ÓöBaCl2ÓŠ°×É«³ĮµķÉś³É |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com