
| �ζ����� | ���������Һ�����mL�� | 0.1000mol/LKMnO4����Һ�����mL�� | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 10.02 |
| �ڶ��� | 25.00 | 0.22 | 11.32 |
| ������ | 25.00 | 1.56 | 11.54 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������Һ | B�����Ը��������Һ |
| C��̼������Һ | D��������ͭ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
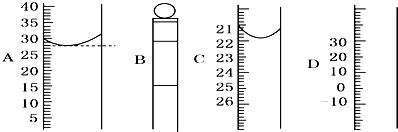
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| 1 |
| 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �ζ����� | �������mL | NaOH��Һ���������ml�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 21.30 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
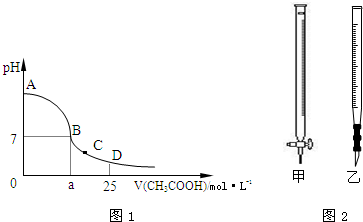
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ��ƿ�е���Һ | �ζ����е���Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ʯ�� | �� |
| B | �� | �� | ���� | �� |
| C | �� | �� | ��̪ | �� |
| D | �� | �� | ��̪ | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
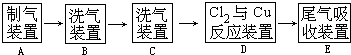
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʵ��˵��������һ�ּ�������ˮ������ |
| B��������ƿ�е�Һ����ɫ����ɫ��Ϊ��ɫ��˵����ˮ�м��� |
| C���γ���Ȫ��ԭ���ǰ�������ˮ����ƿ�ڵ���ѹС�ڴ���ѹ |
| D�����������氱�������ô�װ��Ҳ�ɽ�����Ȫʵ�� |
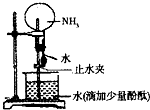
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com