
��15�֣���ͼ����ѧ��ѧ�������ʵ�ת����ϵ��ijЩ��Ӧ���������ֲ�������ȥ����A��GΪ�ճ������еij���������B��C��E��I��JΪ���壬����CΪ����ɫ���壬MΪ���ɫ���塣
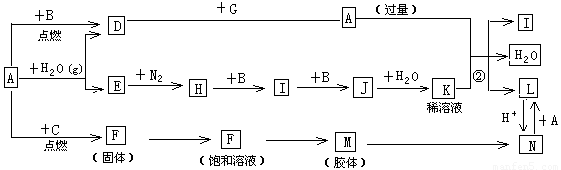
��1����֪AΪ��26��Ԫ�أ���д��AԪ�������ڱ��е�λ��__________��
��2��D��G��Ӧ�Ļ�ѧ����ʽ_________________________��
��3����Ӧ�����ӷ���ʽ__________________________��
��4��F��M�IJ�������_______________��
��5���ֽ�һ�Թ�J���嵹����ˮ����һ��ʱ���ˮ������������������ʹˮ���������Թܣ�Ӧ���Թ���ͨ��һ����__________��������Ļ�ѧʽ������ʱ�Թ�����Һ��Ũ��Ϊ________mol/L�������������״�����㣩��������λ��Ч���֣���
��6����X��Y��Ϊ������Ԫ�أ�X������������C��������Ԫ������������1���Ҳ�ͬ���ڣ�Y��Xͬ���壬���й���X��Y��C��Ԫ��˵����ȷ����__________��
A��ԭ�Ӱ뾶 X��Y��C B�����Ӱ뾶 X��C��Y
C���ǽ����� X��Y��C D������������Ӧˮ�������� C��X��Y
E���⻯��е� X��Y��C
�� ��д��X���⻯����ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ_________��
��7��D��G��Ӧ����A��ͬʱ��������һ�ֲ����ҵ������������Ҫ�ɷ�ΪAl2O3����SiO2��Fe2O3����ȡ��Ĺ����������£�
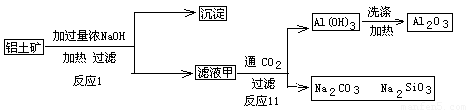
��Ϸ�ӦII���ж�������������ӣ�H+����������ǿ������˳����_________������ĸ��ţ���
A��AlO2�� B��OH�� C��SiO32��
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��J����ѧ��ѧ���������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������֪A��һ�ָ��۵����ʣ�D��һ�ֺ���ɫ���塣
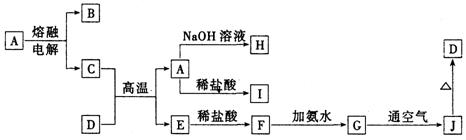
��ش��������⣺
��1��A���ʵ�����Ϊ___________��H��I��Ӧ���������ﻯѧʽΪ ��
��2��C��D�ڸ����µķ�Ӧ��ұ��ҵ�ϳ�Ϊ ��Ӧ�������÷�Ӧ��ʵ�������__________ ______��
��3��G��J�Ļ�ѧ����ʽΪ_________________ __________ _____��
��4��A��H�����ӷ���ʽΪ_________ ____ _______��
��5�������ӷ���ʽ��ʾI���������ھ�ˮ��ԭ��_________ _______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�A��B��C��D��E����ѧ��ѧ������5�ֻ��������A��B�����������X��Y�������г����Ľ�����������ʼ��ת����ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����
��1�����Լ�l���Լ�2������ͬ�����ʣ���X���Լ�l��Ӧ�����ӷ���ʽ�� ��
��2�����Լ�1���Լ�2��ͬ����E��Һ�������ɲ����պ�ɵõ�A����A�Ļ�ѧʽ�� ��
�ټ�������D����Һ�н������ӵ�ʵ������� ��
�ڽ�����C����ˮ������Һ�� ������ԡ��������ԡ����ԡ�����ԭ�������ӷ���ʽ�ɱ�ʾΪ ��
��3����E��Һ���������������ɺ�ɵõ�����Һ�����ʣ���ҵ����E��ϡ�����NaNO2Ϊԭ�����Ʊ���Ч��ˮ��Y��OH��SO4����Ӧ����N0���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ̨����ѧ������ѧ�ڵڶ���ͳ����ѧ�Ծ� ���ͣ������
��14�֣�A��B��C��D��E����ѧ��ѧ������5�ֻ��������A��B�����������X��Y�������г����Ľ�����������ʼ��ת�� ��ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����
��ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����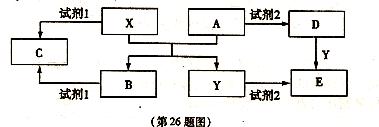
��1�����Լ�l���Լ�2������ͬ�����ʣ���X���Լ�l��Ӧ�����ӷ���ʽ��  ��
��
��2�����Լ�1���Լ�2��ͬ����E��Һ�������ɲ����պ�ɵõ�A����A�Ļ�ѧʽ�� ��
�ټ�������D����Һ�н����� �ӵ�ʵ������� ��
�ӵ�ʵ������� ��
�ڽ�����C����ˮ������Һ�� ������ԡ��������ԡ����ԡ�����ԭ�������ӷ���ʽ�ɱ�ʾΪ ��
��3����E��Һ���������������ɺ�ɵõ�����Һ�����ʣ���ҵ����E��ϡ�����NaNO2Ϊԭ�����Ʊ���Ч��ˮ��Y��OH��SO4����Ӧ����N0���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵڶ���ͳ����ѧ�Ծ� ���ͣ������
��14�֣�A��B��C��D��E����ѧ��ѧ������5�ֻ��������A��B�����������X��Y�������г����Ľ�����������ʼ��ת����ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����
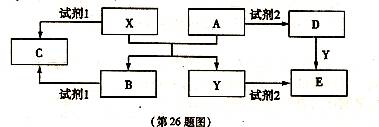
��1�����Լ�l���Լ�2������ͬ�����ʣ���X���Լ�l��Ӧ�����ӷ���ʽ�� ��
��2�����Լ�1���Լ�2��ͬ����E��Һ�������ɲ����պ�ɵõ�A����A�Ļ�ѧʽ�� ��
�ټ�������D����Һ�н������ӵ�ʵ������� ��
�ڽ�����C����ˮ������Һ�� ������ԡ��������ԡ����ԡ�����ԭ�������ӷ���ʽ�ɱ�ʾΪ ��
��3����E��Һ���������������ɺ�ɵõ�����Һ�����ʣ���ҵ����E��ϡ�����NaNO2Ϊԭ�����Ʊ���Ч��ˮ��Y��OH��SO4����Ӧ����N0���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com