
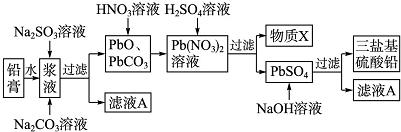
���� ��1�����÷Ͼ�Ǧ����������������Ǧ�ࣩ�Ʊ����ϼӹ����ȶ������λ�����Ǧʵ�����̣���Ǧ�ཬҺ�м���Na2SO3��Һ��Ŀ���ǽ�PbO2��ԭPbO��Na2SO3+PbO2=PbO+Na2SO4����Na2CO3��Һ�ǽ�PbSO4ת����PbCO3��������Һ����Ҫ��Na2SO4��Һ��PbO��PbCO3�������������ת����Pb��NO3����Pb��NO3���м�ϡH2SO4ת����PbSO4�����ᣬ���XΪHNO3����ѭ�����ã��������˵��������ᱵ������ͨ�������Ƿ�������������ӣ�
��2�����������нᾧˮ�ĺ�����ʵ�鲽��Ϊ������ĥ �ڳ�����������װ������������������ �ۼ��� ����ȴ �ݳ��� ���ظ������ݵIJ�����ֱ���������γ��������������0.1gΪֹ �߸���ʵ�����ݼ��㾧���нᾧˮ�ĺ�������������Ĺ����β��������в�������ʯ���������������������ƾ��ơ������ǣ�
��3�����ɵ�����Ǧ������ˮ�������ڹ���PbO��PbCO3�ı��棬�谭��Ӧ�Ľ�һ��������
��4�������̿�������Ǧ���������Ʒ�Ӧ�������λ�����Ǧ�������ƣ��ݴ�д������ʽ��
��5������Ǧ�������������Ǧ�����������ɼ��������Ǧ�����ʵ����������������ƺ�����Ǧ��Ӧ��ϵ�ɼ�����Ҫ�������Ƶ������
��� �⣺��1���������̿�֪��PbO��PbCO3�������������ת����Pb��NO3����Pb��NO3���м�ϡH2SO4ת����PbSO4�����ᣬ���XΪHNO3����ѭ�����ã�������������ӵķ���Ϊ��ȡ����������������ˮ��Ȼ���������ữ���ٵ�BaCl2��Һ�������ְ�ɫ��������֤���þ����к���SO42-��
�ʴ�Ϊ�����ȡ������Һ����������ϡ���ᣬ�ټ��Ȼ�����Һ���۲��Ƿ������ɫ������
��2�����������нᾧˮ�ĺ�����ʵ�鲽��Ϊ������ĥ �ڳ�����������װ������������������ �ۼ��� ����ȴ �ݳ��� ���ظ������ݵIJ�����ֱ���������γ��������������0.1gΪֹ �߸���ʵ�����ݼ��㾧���нᾧˮ�ĺ�������������Ĺ����β��������в�������ʯ���������������������ƾ��ơ������ǣ�
�ʴ�Ϊ���������ƾ��ƣ������ǣ�
��3�����ɵ�����Ǧ������ˮ�������ڹ���PbO��PbCO3�ı��棬�谭��Ӧ�Ľ�һ���������ʴ�Ϊ��PbSO4������ˮ�������ڹ�������谭��Ӧ�Ľ�һ��������
��4�������̿�������Ǧ���������Ʒ�Ӧ�������λ�����Ǧ�������ƣ���Ӧ����ʽΪ��4PbSO4+6NaOH=3PbO•PbSO4•H2O+3Na2SO4+2H2O�����ӷ���ʽΪ��
4PbSO4+6OH-=3PbO•PbSO4•H2O+3SO42-+2H2O���ʴ�Ϊ��4PbSO4+6OH-=3PbO•PbSO4•H2O+3SO42-+2H2O��
��5������Ǧ�����ʵ���Ϊ��$\frac{47.8g��15%}{239g/mol}$=0.03mol��
PbO2 ��Na2SO3
1mol 1mol
0.03mol n
n=0.03mol��V=$\frac{0.03mol}{1.0mol/L}$=0.03L=30mL��
�ʴ�Ϊ��30��
���� ���⿼����ʵ�鷽������ƣ��е��Ѷȣ�Ҫ�������̣���������������Ϣ�����Ŀ���ʽ��⣮


 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ѹǿ/MPa ת����/% �¶�/�� | 0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
| 500 | 93.5 | 96.9 | 97.8 | 99.3 |
| 600 | 73.7 | 85.8 | 89.5 | 96.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2���������� | |
| B�� | CuFeS2������ԭ������Ԫ�ر����� | |
| C�� | ÿ����1mol Cu2S����4mol������ | |
| D�� | ÿת��1.2mol���ӣ���0.2mol������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� �� | �� | �� | �� | �� |
| ϡ�������/mL | 100mL | 200mL | 300mL | 400mL |
| ʣ�����/g | 18.0g | 9.6g | 0 | 0 |
| NO���/L����״���£� | 2.24L | 4.48L | 6.72L | V |
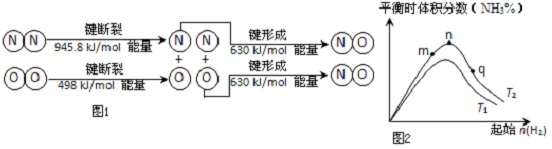
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
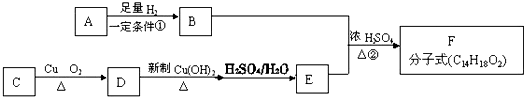
 ��
�� ��
�� ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��֬ˮ��ɵõ���������� | |
| B�� | ����������һ���Ķ��ԣ����Բ��ܳԺ���������ʳƷ | |
| C�� | ��������Һ��������Һ�������� | |
| D�� | �����������͵����ǽ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Һ�������Һ��ϣ�SiO32-+2H+=H2SiO3�� | |
| B�� | NH4Al��SO4��2��Һ�����ϡ��ˮ��Ӧ��Al3++3NH3•H2O=Al��OH��3��+3NH4+ | |
| C�� | ��ϡ������ϴ�Թ��ڱڵ�������Ag+2H++NO3-=Ag++NO2��+H2O | |
| D�� | FeBr2��Һ��ͨ�����Cl2��2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com