ŅŃÖŖ25”ꏱ²æ·ÖČõµē½āÖŹµÄµēĄėĘ½ŗā³£ŹżŹż¾ŻČē±ķĖłŹ¾£ŗ
| »ÆѧŹ½ | CH3COOH | H2CO3 | HClO |
| µēĄėĘ½ŗā³£Źż | Ka=1.8”Į10-5 | Kal=4.3”Į10-7 | Ka2=5.6”Į10-11 | Ka=3.0”Į10-8 |
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĪļÖŹµÄĮæÅØ¶Č¾łĪŖ0.1mol?L
-1µÄĖÄÖÖČÜŅŗ£»
a£®CH
3COONa b£®Na
2CO
3 c£®NaClO d£®NaHCO
3pHÓÉŠ”µ½“óÅÅĮŠµÄĖ³ŠņŹĒ
£ØÓƱąŗÅĢīŠ“£©£®
£Ø2£©³£ĪĀĻĀ£¬0.1mol?L
-1 CH
3COOHČÜŅŗ¼ÓĖ®Ļ”ŹĶ¹ż³ĢÖŠ£¬ĻĀĮŠ±ķ“ļŹ½µÄŹż¾Ż±ä“óµÄŹĒ
£®
A£®c£ØH
+£©
B£®
C£®c£ØH
+£©?c£ØOH
-£©
D£®
E£®
| c(H+)?c(CH3COO-) |
| c(CH3COOH) |
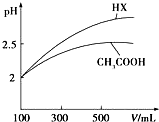
£Ø3£©Ģå»ż¾łĪŖ100mL pH=2µÄCH
3COOHÓėŅ»ŌŖĖįHX£¬¼ÓĖ®Ļ”ŹĶ¹ż³ĢÖŠpHÓėČÜŅŗĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŌņĶ¬ĪĀ¶ČŹ±HXµÄµēĄėĘ½ŗā³£Źż
£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©CH
3COOHµÄµēĄėĘ½ŗā³£Źż£¬ĄķÓÉŹĒ
£®
£Ø4£©25”ꏱ£¬CH
3COOHÓėCH
3COONaµÄ»ģŗĻČÜŅŗ£¬Čō²āµĆpH=6£¬ŌņČÜŅŗÖŠc£ØCH
3COO
-£©-c£ØNa
+£©=
mol?L
-1£ØĢī¾«Č·Öµ£©£®
£Ø5£©±ź×¼×“æöĻĀ£¬½«1.12L CO
2ĶØČė100mL 0.75mol?L
-1µÄNaOHČÜŅŗÖŠ£¬ŌņČÜŅŗÖŠĄė×ÓµÄÅضČÓɓ󵽊”µÄĖ³Šņ
£®

 ŅŃÖŖ25”ꏱ²æ·ÖČõµē½āÖŹµÄµēĄėĘ½ŗā³£ŹżŹż¾ŻČē±ķĖłŹ¾£ŗ
ŅŃÖŖ25”ꏱ²æ·ÖČõµē½āÖŹµÄµēĄėĘ½ŗā³£ŹżŹż¾ŻČē±ķĖłŹ¾£ŗ

 ĶõŗóŠŪѧ°ø½Ģ²ÄĶźČ«½ā¶ĮĻµĮŠ“š°ø
ĶõŗóŠŪѧ°ø½Ģ²ÄĶźČ«½ā¶ĮĻµĮŠ“š°ø
