
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�
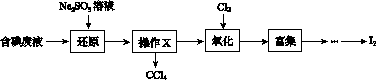
(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
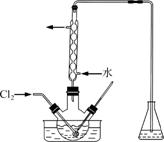
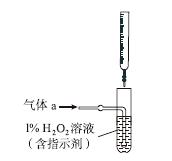
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��
(4)��֪��5SO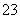 ��2IO
��2IO ��2H��===I2��5SO
��2H��===I2��5SO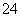 ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
(1)SO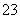 ��I2��H2O===2I����SO
��I2��H2O===2I����SO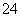 ��2H����ʹCCl4�еĵ����ˮ��
��2H����ʹCCl4�еĵ����ˮ��
(2)��Һ
(3)ʹ��������Һ���нϴ���ܽ��(���ֹI2�������ֹI2��һ��������)��NaOH��Һ
(4)��ˮ��ȡ������Һ������1��2 mL������Һ���������ữ���μ�FeCl3��Һ������Һ������˵����ˮ�к���I��������Һ��������˵����ˮ�в�����I��������ˮ��ȡ������Һ������1��2 mL������Һ���������ữ���μ�Na2SO3��Һ������Һ������˵����ˮ�к���IO ������Һ��������˵����ˮ�в�����IO
������Һ��������˵����ˮ�в�����IO
[����] (1)SO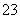 ��I2����ΪSO
��I2����ΪSO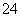 ��I2����ԭΪI������ϵ���غ��ԭ���غ�ɵ�SO
��I2����ԭΪI������ϵ���غ��ԭ���غ�ɵ�SO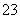 ��I2��H2O===2I����SO
��I2��H2O===2I����SO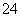 ��2H������ΪI2������ˮ�����⻯��������ˮ���ʽ�I2��ԭΪI����Ŀ����ʹ��Ԫ�ؽ���ˮ�㡣(2)�����л��ܼ���ˮ��Һ�Ļ������Ҫ��Һ��(3)�¶�Խ�ߣ�Cl2�ܽ��ԽС���������¶����ߣ�Cl2���I2��һ������ΪIO
��2H������ΪI2������ˮ�����⻯��������ˮ���ʽ�I2��ԭΪI����Ŀ����ʹ��Ԫ�ؽ���ˮ�㡣(2)�����л��ܼ���ˮ��Һ�Ļ������Ҫ��Һ��(3)�¶�Խ�ߣ�Cl2�ܽ��ԽС���������¶����ߣ�Cl2���I2��һ������ΪIO ����������I����Ч��ƫ�ͣ����⣬I2Ҳ����������
����������I����Ч��ƫ�ͣ����⣬I2Ҳ����������
(4)����I2�õ��ۣ���������Լ������ʣ�FeCl3���������ԣ��ɽ�I������ΪI2����Na2SO3����ǿ��ԭ�ԣ��ɽ�IO ��ԭΪI2��
��ԭΪI2��


 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������ֺ��϶࣬���ȵ�������ڷ����µ���Ա����Ҫ�Ĵ�ʩ�Ǹ���Ա������������ͼ��ҽԺ����Ա��Һʱ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ��ǩ��������۲��ǩ�����е����ݺ���д��

(1)�����ǵ�Ħ������Ϊ________��
(2)����Һ�� ��ˮ________ g��
��ˮ________ g��
(3)����Һ���ܶ�ԼΪ________ g/mL��
(4)����Һ�����ʵ���Ũ��Ϊ________ mol/L(��ȷ��С���������λ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں�ۡ��ۡ�����֮�佨����ϵ���ǻ� ѧѧ�����е�˼ά��ʽ������β������ɴ�����Ⱦ����Ҫԭ��֮һ���������������ϰ�װ����ת�����������ʹ������β��ת���������塣���á��ʾ̼ԭ�ӣ��á��ʾ��ԭ�ӣ���
ѧѧ�����е�˼ά��ʽ������β������ɴ�����Ⱦ����Ҫԭ��֮һ���������������ϰ�װ����ת�����������ʹ������β��ת���������塣���á��ʾ̼ԭ�ӣ��á��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
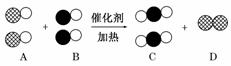
(1)A��B��C�������ʿ��Թ�Ϊһ���������___________________________________��
(2)��C��Ϊ�������D��Ϊ���ʵ�������________________________________
________________________________________________________________________��
(3)�û�ѧ��Ӧ����ʽ��ʾΪ___________________________________________
________________________________________________________________________��
��ѧ�仯���������ĵ�A���ʺ����ɵ�C���ʵ�������Ϊ________��
(4)���۵ĽǶ�ȥ�������õĹ��ڻ�ѧ�仯���й���Ϣ(���һ������)________________________________________________________________________
__________________________________________________ ______________________��
______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼����ĺ���Ӱ��������ܡ�̼��������һ�ֲⶨ�����ǽ������е�̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
(1)����װ��A���ڸ����½�x g�����е�̼����ת��ΪCO2��SO2��

������a�ijɷ���______��
��������������FeS��ʽ���ڣ�A�з�Ӧ��
3FeS��5O2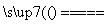 1________��3________��
1________��3________��
(2)������aͨ�����װ����(��ͼ)�����õζ����ⶨ��ĺ�����
��H2O2����SO2�Ļ�ѧ����ʽ��__________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mL NaOH��Һ��������1 mL NaOH��Һ�൱���������Ϊy g����ø������������������________��
(3)������aͨ���̼װ����(��ͼ)�������������ⶨ̼�ĺ�����
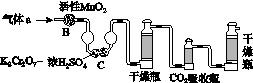
������aͨ��B��C��Ŀ����________________________________________��
�ڼ��������̼������������Ӧ������������_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�弰�仯����㷺Ӧ����ҽҩ��ũҩ����ά��������ȼ���ȣ��ش��������⣺
(1)��ˮ��������У���Ũ���ĺ�ˮ��ͨ��________�������е�Br�����������ÿ��������壻Ȼ����̼������Һ�����壬���绯ΪBr����BrO �������ӷ���ʽΪ__________________________________________��
�������ӷ���ʽΪ__________________________________________��
(2)���������Թ��ۼ�����γ�BrCl��BrCl�����У�________�������ԡ�BrCl��ˮ������Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
(3)CuBr2�ֽ���Ȼ�ѧ����ʽΪ��
2CuBr2(s)===2CuBr(s)�� Br2(g)
��H����105.4 kJ/mol
���ܱ������н�����CuBr2��487 K�¼��ȷֽ⣬ƽ��ʱp(Br2)Ϊ4.66��103 Pa��
���練Ӧ��ϵ��������䣬��߷�Ӧ�¶ȣ���p(Br2)����________(��������䡱��С��)��
���練Ӧ�¶Ȳ��䣬����Ӧ��ϵ���������һ������p(Br2)�ı仯��ΧΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���û�з�����ѧ��Ӧ����(����)
A���û���̿ȥ�������е���ζ
B�����ȼ�ˮ��������ϲ���������
C���ý��ݹ����������Һ�Ĺ���������ˮ��
D���ú��轺�����۵���С����ʳƷһ���ܷ��װ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ����ȷ����(����)
A��������Һ�м�������������þ���壺Mg(OH)2��2CH3COOH===Mg2����2CH3COO����2H2O
B��H2O2��Һ�м�����������KMnO4��Һ��2MnO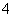 ��3H2O2��6H��===2Mn2����6H2O��4O2��
��3H2O2��6H��===2Mn2����6H2O��4O2��
C��Ca(HCO3)2��Һ�м�����������ʯ��ˮ��Ca2����HCO ��OH��===CaCO3����H2O
��OH��===CaCO3����H2O
D��NH4HSO4��ϡ��Һ����μ���Ba(OH)2��Һ��SO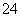 ǡ�ó�����ȫ��NH
ǡ�ó�����ȫ��NH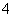 ��H����SO
��H����SO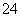 ��Ba2����2OH��===NH3��H2O��BaSO4����H2O
��Ba2����2OH��===NH3��H2O��BaSO4����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��Ӧ��ʵ���ǡ��ɼ��Ķ��Ѻ��¼����γɡ�����һ�������£�һ���Ҵ����Ӷϼ�ֻʧȥ������ԭ�ӣ����ɵ����л������������(д�ṹ��ʽ����һ������)_______________��__________��______________��________________��������ѧϰ����֪ʶ���ṩ���Լ���������ʵ������ijһ��ת��������֤��ת������л�������Լ�����ˮ�Ҵ���������ͭ˿������������Һ������ͭ��Һ���������Թܡ��ԹܼС����ӡ���ͷ�ιܡ��ƾ��ơ����
(1)�Ҵ���ת����
��ʵ�鷽����_____________________________________��
��ʵ��������_____________________________________��
���Ҵ�ת���Ļ�ѧ����ʽ________________________��
(2)�������֤��
�پ������л����������Ҫ���ʵ�ԭ���ŵĽṹʽ��________��������______________��
����֪�ṹ���Ƶ����ʣ��������ƵĻ�ѧ���ʡ�������һԭ����֤���Ҵ�ת���ɵ��л�����������Լ�(д�Լ�����)__________ _��Ȼ���Լ��뱻��֤���л��������ϣ������������ڣ��۲쵽��������______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����(����)
A����Ƭ��NaOH��Һ��Ӧ��Al��2OH��===AlO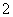 ��H2��
��H2��
B��ϡ�����м�������̼��������FeCO3��2H��===Fe2����CO2����H2O
C������̼������Һ��ͨ�������̼���壺2Na����CO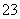 ��CO2��H2O===2NaHCO3��
��CO2��H2O===2NaHCO3��
 D��Ba(OH)2�������NH4HSO4��Ӧ��Ba2����2OH����2H����SO
D��Ba(OH)2�������NH4HSO4��Ӧ��Ba2����2OH����2H����SO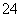 ===BaSO4����2H2O
===BaSO4����2H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com