
��AΪԭ���ڻ�ѧ��ҵ�Ͽ��Ʊ��������ʣ����е�һ������ͼ��ʾ��A��B��D��E��G����ɫ��Ӧ���ʻ�ɫ��A����ͬ����Ԫ����ɵ����ӻ������ˮ��Һ�����ԣ�B��D��E��ˮ��Һ���ʼ��ԡ������£�X2Ϊ����ɫ���塣��Gת��ΪD��Eʱ��ͬʱ������֧��ȼ�յ���ɫ����Y2��

�Իش��������⣺
��1��G�Ļ�ѧʽΪ ��A�ĵ���ʽΪ ��E�еĻ�ѧ������Ϊ ��
��2����ҵ����AΪԭ����ȡE�����ӷ���ʽΪ ��
��3��B��Һ�ʼ��Ե������ǣ������ӷ���ʽ��ʾ�� ��
��4��D�ڹ�ҵ�Ͽ����ڼ��Ậ��ȼ��ȼ��ʱ��SO2��Ⱦ����D��ĩ��ȼ��һ�����ȼ¯��D��SO2��Ӧ�������壨�ù���ɽ�һ��������������Ӧ�Ļ�ѧ����ʽΪ
��
��5����X��Y����Ԫ����ɵ�ij�ֻ������У�YԪ�ص���������Ϊ47.4%���û�������NaOH��Ӧ�������ֺ������Σ�����X�Ļ�����ֱ�Ϊ+3�ۺ�+5�ۣ���Ӧ�Ļ�ѧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
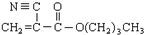 ��
��
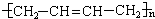 ����Ӧ�ķ�Ӧ���ͷֱ�Ϊ
����Ӧ�ķ�Ӧ���ͷֱ�Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������������2007������꼶�������������ۺϻ�ѧ���� ���ͣ�022
| |||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�Ͱ���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
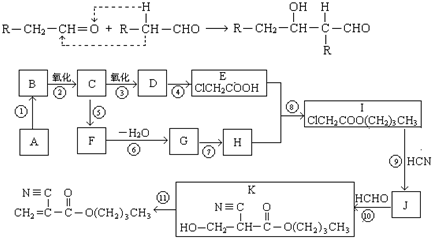
��12�֣�ú���ۺ������ǵ�����ѧ��ҵ�����ƣ���úΪԭ�Ͽɺϳ���Ҫ�����м���G������B�����ụΪͬ���� ���壬����Na��Ӧ�ų�������
���壬����Na��Ӧ�ų�������
��֪���� CH3CHO + CH3CHO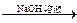
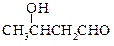
��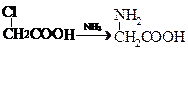 ���ʰ��ᣩ
���ʰ��ᣩ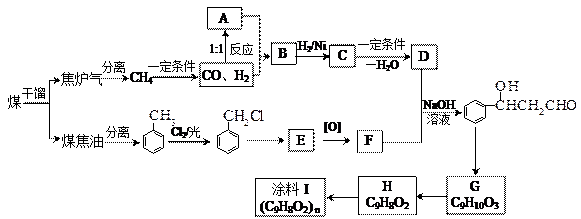
�ش��������⣺
��1��ú�ĸ�������____________�仯��ʵ���Ҽ���D���õ��Լ�Ϊ________________________��
��2��A��F�Ľṹ��ʽ�ֱ�Ϊ_____________________��______________________________��
��3��д��B��C�Ļ�ѧ����ʽ___________________________________________________��
H��I�Ļ�ѧ����ʽ___________________________________________________��
��4������Na2CO3��Һ��Ӧ��E�ķ����廯�����ͬ���칹����________________�֡�
��5��������֪��Ϣ����һ����������G�ϳ�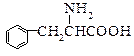 ������Ҫ_______����ѧ��Ӧ��
������Ҫ_______����ѧ��Ӧ��
a��3 b��4 c��5 d��6
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com