
����Ŀ�������ǵ���N2O����һ��ǿ�������壬����ת���ɿ�����Ⱦ��о������ǵ��ֽ�Ի�����������Ҫ���塣
��1����ˮ�����ѵ������У�������������£�����刺ɷֽ�ΪN2O����һ�ֲ���÷�Ӧ�Ļ�ѧ����ʽΪ________��
��2����֪��Ӧ2N2O(g)=2N2(g) + O2(g)�Ħ�H= �C163 kJ��mol��1��1molN2(g)��1molO2(g)�����л�ѧ������ʱ�ֱ���Ҫ����945 kJ��498 kJ����������1molN2O(g)�����л�ѧ������ʱ��Ҫ���յ�����Ϊ________ kJ��
��3����һ���¶��µĺ��������У���Ӧ2N2O(g)=2N2(g) + O2(g)�IJ���ʵ���������£�
��Ӧʱ��/min | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
C(N2O)/mol/L | 0.100 | 0.090 | 0.080 | 0.070 | 0.060 | 0.050 | 0.040 | 0.030 | 0.020 | 0.010 | 0.010 |
����0��20minʱ�Σ���Ӧ����v(N2O)Ϊ________ mol��L��1��min��1��
����N2O��ʼŨ��c0Ϊ0.150 mol��L��1����Ӧ��30minʱN2O��ת���ʦ� =__________���Ƚϲ�ͬ��ʼŨ��ʱN2O�ķֽ����ʣ�v(c0=0.150 mol��L��1) ________ v(c0=0.100 mol��L��1)���>������=����<������
�۲�ͬ�¶ȣ�T���£�N2O�ֽ��˥������ʼѹǿ�ı仯��ϵ��ͼ��ʾ��ͼ�а�˥��ָ��һŨ��N2O����һ��ʱ�������Ӧʱ�䣩����T1________T2���>������=����<���������¶�ΪT1����ʼѹǿΪp0����Ӧ��t1 minʱ����ϵѹǿp =________����p0��ʾ����

��4�������������ܴ�������N2O�ķֽ����ʣ���Ӧ����Ϊ��
��һ�� I2(g) ![]() 2I(g) ���췴Ӧ��
2I(g) ���췴Ӧ��
�ڶ��� I(g)+N2O(g)��N2(g)+IO(g) ������Ӧ��
������ IO(g)+N2O(g)��N2(g)+O2(g)+I(g) ���췴Ӧ��
ʵ�����������ʱN2O�ֽ����ʷ���v=k��c(N2O)��[c(I2)]0.5��kΪ���ʳ����������б�����ȷ����________������)��
A��N2O�ֽⷴӦ�У�k�����⣩> k���⣩ B����һ�����ܷ�Ӧ�������������
C���ڶ�����ܱȵ������� D��I2Ũ����N2O�ֽ�������
���𰸡�NH4NO3![]() N2O+2H2O 1112.5 1.0��10-3 20.0% = > 1.25p0 AC
N2O+2H2O 1112.5 1.0��10-3 20.0% = > 1.25p0 AC
��������
��1������刺ɷֽ�ΪN2O����һ�ֲ�����������غ㶨�ɿ��ж���һ����ΪH2O�����ݵ�ʧ�����غ�д����Ӧ�Ļ�ѧ����ʽ��
��2������ӦΪ���ȷ�Ӧ����ѧ������ʱ�ų�������-��ѧ������ʱ�����յ�����=��Ӧ�ų����������Դ˷������
��3���ٸ��ݷ�Ӧ����v(N2O)=![]() ���㣻
���㣻
�ڴӱ������ݷ�����֪��ÿ��ʱ����ڣ���c(N2O)��ȣ���v(N2O)��ȣ�����ʼŨ���أ���N2O��ʼŨ��c0Ϊ0.150 mol��L��1����Ӧ��30minʱN2Oת����Ũ��Ϊ0.010mol/L��3=0.030mol/L����ת���ʦ� =![]() ��100%=20.0%��
��100%=20.0%��
���¶�Խ�߷�ӦԽ�죬��˥��Խ�̣���ͼ��֪��ѹǿһ��ʱ��T1ʱ��˥��С��T2����T1>T2������������ͬʱ��ѹǿ֮�ȵ������ʵ���֮�ȣ��ɷ�Ӧʽ2N2O(g)=2N2(g)+ O2(g)��֪������ʼʱ��2molN2O������һ��ֽ�ʱ������1molN2��0.5molO2����ʣ��1molN2O����ʱ������������ʵ�����Ϊ��1mol+1mol+0.5mol��=2.5mol������ϵѹǿp =![]() P0=1.25p0��
P0=1.25p0��
��4����ѧ��Ӧ���ʿ���ȡ���ڷ�Ӧ���������ķ�Ӧ�����ڶ�����Ӧ���÷�Ӧ���ܷ�ӦΪ2N2O(g)=2N2(g)+O2(g)�����ڷ�Ӧ����������ã��Դ˷������
��1������刺ɷֽ�ΪN2O����һ�ֲ�����������غ㶨�ɣ����ж���һ����ΪH2O����Ӧ�Ļ�ѧ����ʽΪNH4NO3![]() N2O+2H2O��
N2O+2H2O��
�ʴ�Ϊ��NH4NO3![]() N2O+2H2O��
N2O+2H2O��
��2������ӦΪ���ȷ�Ӧ����ѧ������ʱ�ų�������-��ѧ������ʱ�����յ�����=��Ӧ�ų�����������1molN2O(g)�����л�ѧ������ʱ��Ҫ���յ�����ΪxkJ����945 kJ��2+498 kJ-2x kJ =163 kJ�����x=1112.5��
�ʴ�Ϊ��1112.5��
��3������0��20minʱ�Σ�v(N2O)=![]() =
=![]() =1.0��10-3 mol��L��1��min��1��
=1.0��10-3 mol��L��1��min��1��
�ڴӱ������ݷ�����֪��ÿ��ʱ����ڣ���c(N2O)��ȣ���v(N2O)��ȣ�����ʼŨ���أ����Բ�ͬ��ʼŨ��ʱN2O�ķֽ����ʣ�v(c0=0.150 mol��L��1) = v(c0=0.100 mol��L��1)����N2O��ʼŨ��c0Ϊ0.150 mol��L��1����Ӧ��30minʱN2Oת����Ũ��Ϊ0.010mol/L��3=0.030mol/L����ת���ʦ�=![]() ��100%=20.0%��
��100%=20.0%��
�ʴ�Ϊ��20.0% ��=��
���¶�Խ�߷�ӦԽ�죬��˥��Խ�̣���ͼ��֪��ѹǿһ��ʱ��T1ʱ��˥��С��T2����T1>T2������������ͬʱ��ѹǿ֮�ȵ������ʵ���֮�ȣ��ɷ�Ӧʽ2N2O(g)=2N2(g) + O2(g)��֪������ʼʱ��2molN2O������һ��ֽ�ʱ������1molN2��0.5molO2����ʣ��1molN2O����ʱ������������ʵ�����Ϊ��1mol+1mol+0.5mol��=2.5mol������ϵѹǿp =![]() P0=1.25p0��
P0=1.25p0��
�ʴ�Ϊ��> ��1.25p0��
��4����ѧ��Ӧ���ʿ���ȡ���ڷ�Ӧ���������ķ�Ӧ�����ڶ�����Ӧ���÷�Ӧ���ܷ�ӦΪ2N2O(g)=2N2(g) + O2(g)�����ڷ�Ӧ����������ã�
A�����ڷ�Ӧ����������ã�N2O�ֽⷴӦ�У�k�����⣩> k���⣩����A��ȷ��
B���ڶ�����ӦΪ����Ӧ�����ܷ�Ӧ������������ã���B����
C���ڶ�����ӦΪ����Ӧ����������ӦΪ�췴Ӧ���ڶ�����ܱȵ�������C��ȷ��
D���ɺ���ʱN2O�ֽ����ʷ���v=k��c(N2O)��[c(I2)]0.5��֪�� N2O�ֽ�������[c(I2)]0.5�����ȣ���D����ѡAC��
�ʴ�Ϊ��AC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���У�������ǣ� ��
A.ԭ�Ӽ������ӵĺ�����Ӳ������ڸ�Ԫ�����ڵ���������
B.Ԫ�����ڱ��дӢ�B�嵽��B��10�����е�Ԫ�ض��ǽ���Ԫ��
C.��He�������ϡ������Ԫ��ԭ�ӵ���������������8
D.��A�������֮�����10������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�����������˵������ȷ���ǣ�������
A. ��״���£�22.4L CO��11.2L O2��Ϻ���������ԼΪ22.4L
B. ��״���£�22.4 L CCl4�����ķ�����ΪNA
C. 300 mL 0.2 mol/L������Һ������������Ϊ0.06NA
D. 14 g����ϩ(C2H4)�ͱ�ϩ(C3H6)��ɵĻ�����к���ԭ�ӵ���ĿΪ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ش��������⡣
��1����������10�����ʣ�
�ٴ��� ��Mn2O7 ������ ��CaO ��CO2 ��С�մ� ��K2SO3 ��SO2 ��Na2O2 ��CO��
�������������__________�������������������_________��
��2��ʵ�����Ʊ�Fe(OH)3����Ļ�ѧ����ʽΪ___________________,ȡ����Fe(OH)3�������Թ��У���μ����������������ɿ�����������___________________�����ٺ�ɫ������(Sb2S3)���壬װ��U�ιܣ�����缫��ֱͨ���磬�������������ٺ�ɫ���֤��Sb2S3������____(���������)��ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�����ؼ�����գ�
��1���ڱ�״���£�1.6 g ij������RO3���Ϊ0.448L����R�����ԭ����Ϊ________��
��2����֪Wg����A����a�����ӣ���ô�ڱ�״���£�bg����A��ռ�������________�������а����ӵ�������NA��ʾ��
��3����״���£��ܶ�Ϊ0.75g/L��NH3��CH4��ɵĻ�������У�NH3���������Ϊ________��
��4��VmLAl2(SO4)3��Һ�к�Al3+ ag��ȡV/5 mL��Һϡ�͵�VmL����ϡ�ͺ���Һ�е�SO42�������ʵ���Ũ����______________ ��
��5��10 mL 0.1 mol��L��1 BaCl2��Һǡ���ֱܷ�ʹ��ͬ�����������������ͭ������Һ�е�SO42����ȫת���ɳ�������������������ͭ������Һ�����ʵ���Ũ��֮����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ػ����Һ��Ϊȼ�ռ���Һ��Ϊ����������֪:
��H2(g)=H2(l)����H=-0. 92 kJ��mol-1 ��O2(g)=O2(l)����H=-6. 84 kJ��mol-1
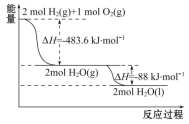
����˵����ȷ����
A. 2 mol H2(g)��1 mol O2(g)�������������2 mol H2O(g)�������������
B. ������ȼ����Ϊ��H=-241. 8 kJ��mol-1
C. �����Һ��ȼ�յ��Ȼ�ѧ����ʽΪ2H2(l)+O2(l)![]() 2H2O(g)����H=-474. 92 kJ��mol-1
2H2O(g)����H=-474. 92 kJ��mol-1
D. H2O(g)���H2O(l)�Ĺ����У��ϼ����յ�����С�ڳɼ��ų�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�����������������ڷ�Ӧ�����������ǣ� ��
A.̼������ȷֽ�B.�Ҵ�ȼ��
C.��������������ĩ��ӦD.����������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NaAlH4(��������)���л��ϳɵ���Ҫ��ԭ����ijС�������Ͻķϱ߽���Ϊԭ��(��Ҫ�ɷ�ΪAl����������Al2O3��Fe2O3��MgO��SiO2���������Ʊ��������ƵĹ����������£�

������ʾ��NaH��NaAlH4��ˮ�����������ҷ�Ӧ��
��ش��������⣺
(1) NaAlH4����Ԫ�صĻ��ϼ�Ϊ___________��
(2)�Լ�A�����ʵ�����������������������ȣ������ʽΪ___________���ڿ�������������2���õ��Ĺ������Ҫ�ɷ�Ϊ___________(�ѧʽ)��
(3)��Һ3����ѭ�����ã�д����Һ2����Һ3��Ӧ�����ӷ���ʽ��___________��
(4)ʵ��ǰҪ��֤NaH��AlC13��Ӧ��װ�ø����Ŀ����___________________��
(5)��֪���ϽӴ�������Ӵ�ʱ�����ͬ�������������Ԫ�ؽ�����������Ũ�ȵĹ�ϵ��ͼ��ʾ��������Ũ�ȴ��� C0 mol��L��1ʱ�������ʽ��͵�ԭ�������___________��
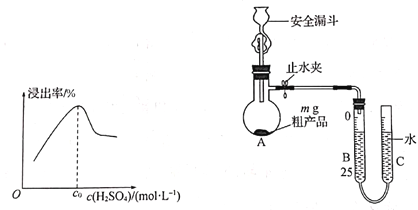
(6)�ⶨNaAlH4�ֲ�Ʒ�Ĵ��ȡ�
��ȡ m g NaAlH4�ֲ�Ʒ����ͼ��ʾװ�ý���ʵ�飬�ⶨ��Ʒ�Ĵ��ȡ�
������ȫ©����������ȫ���ĺ�����____________________________________________��
����֪ʵ��ǰB�ܶ���ΪxmL����A�м�����������ˮ����A�з�Ӧ��ȫ����ȴ������B�ܶ���ΪymL(���ۺϳɱ�״��)����ò�Ʒ�Ĵ���Ϊ___________(�ú�m��x��y�Ĵ���ʽ��ʾ)��
����ʵ��ǰ����ʱB�ܺ�C��Һ����ƽ��ʵ������ʱB��Һ�����C�ܣ����õĽ��___________(����ƫ������ƫ����������Ӱ����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������û�ѧ��Ӧԭ�������֪ʶ�о�̼���仯��������ʡ�
(1)�ҹ���������̼���о�ȡ���ش��չ���õ绡���ϳɵ�̼�����г�����̼������(����)������̼���������������������ķ����ᴿ��������ʹ���Ϊ���������ȵ��ظ����(K2Cr2O7)������Һ���ݷ�����Ӧ����Һ���dz��ɫ(Cr3+)��ͬʱ����ʹ����ʯ��ˮ����ǵ����壬д����Ӧ�����ӷ���ʽ��____________________________________________��
(2)��̿��������ȡˮú�������24g̼��ˮ������ȫ��Ӧ����ˮú��ʱ��������263.2kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ_________________________________��
(3)�״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪCO(g)+2H2(g) ![]() CH3OH(g) ��H1=��116kJ��mol��1��
CH3OH(g) ��H1=��116kJ��mol��1��
�����д�ʩ��������߷�Ӧ��ת���ʵ���___________(�����)��
A.��ʱ��CH3OH�뷴Ӧ�������� B.���ͷ�Ӧ�¶�
C.��С��ϵѹǿ D.ʹ�ø�Ч����
���Ҵ���ؾ��кܸߵ�ʵ�ü�ֵ������ͼ��ʾ��һ������ȼ�ϵ�ؾƾ�����ǣ������Զ����������������ƵĹ��ܣ��dz��ʺϽ����ֳ��ƾ���⡣�õ�صĸ�����ӦʽΪ_________________________________��

����֪��CO(g)+![]() O2(g)=CO2(g) ��H2=��283 kJ��mol��1
O2(g)=CO2(g) ��H2=��283 kJ��mol��1
H2(g)+ ![]() O2(g)=H2O(g) ��H3=? kJ��mol��1
O2(g)=H2O(g) ��H3=? kJ��mol��1
1mol��̬�״���ȫȼ������CO2��ˮ����ʱ�ų�����651kJ�����H3=________________��
�����ݻ�Ϊ1L�ĺ��������У��ֱ��о���230�桢250���270�������¶��ºϳɼ״��Ĺ��ɣ���ͼ�����������¶��²�ͬ��H2��CO����ʼ��ɱ�(��ʼʱCO�����ʵ�����Ϊ1mol)��COƽ��ת���ʵĹ�ϵ����ش��������⣺

(i)�����������¶��У�����X��Ӧ���¶���______________________��
(ii)����ͼ��a���Ӧ�����ݣ����������Z�ڶ�Ӧ�¶���CO(g)+2H2(g)![]() CH3OH(g)��ƽ�ⳣ��K=___________��
CH3OH(g)��ƽ�ⳣ��K=___________��
(4)CO2����������������Һ�����գ�����0.2moCO2������100mL3 mol/LNaOH��Һ������ȫ���գ���Һ������Ũ���ɴ�С��˳��Ϊ______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com