
 ����Na2CO4�������ϴ����������Ŀ���Ƕ��������Ư�ס����������û�ѧ����ʽ��ʾ����ϴ��ԭ���� ��
����Na2CO4�������ϴ����������Ŀ���Ƕ��������Ư�ס����������û�ѧ����ʽ��ʾ����ϴ��ԭ���� ��| A��MnO2 | B��H2S | C��CH3COOH | D��NaHCO3 |
 ��
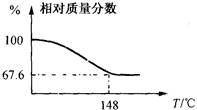
�� ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��̼���ƣ�Na2CO3��10H2O������ g��
��̼���ƣ�Na2CO3��10H2O������ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��������ȡ�����Ҫװ������:
��������ȡ�����Ҫװ������: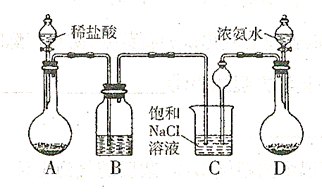
 ,�����D������(ֻ��һ�� ��Cװ��������©���������� ��
,�����D������(ֻ��һ�� ��Cװ��������©���������� �� ������ܺ��е������� ���ø�
������ܺ��е������� ���ø�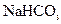 ������ȡ����ʱ���е����ʲ���Ӱ�촿��Ĵ��ȡ�ԭ����
������ȡ����ʱ���е����ʲ���Ӱ�촿��Ĵ��ȡ�ԭ���� 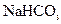 ��ȡ����Ļ�ѧ����ʽ ��
��ȡ����Ļ�ѧ����ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��20% | B��30% | C��45% | D�� 55%�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
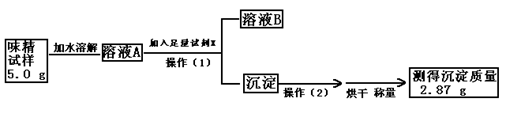 [
[ �� ��
�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��P1=P2 | B��P1=2P2 | C��P1=4P2 | D��P1=8P2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
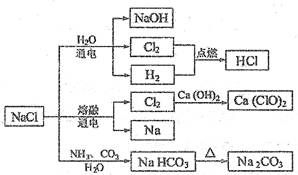
| A������ʱ��NaHCO3��ˮ�е��ܽ�ȱ�Na2CO3�Ĵ� |
| B��ʯ������Cl2�ķ�Ӧ�������Ʊ�Ư�ۣ�Ư�۵���Ҫ�ɷ���Ca(ClO)2��CaCl2 |
| C�������¸����Cl2���ø�ƿ���棬����Cl2��������Ӧ |
| D��ͼ����ʾת����Ӧ����������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com