
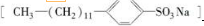 �dz���ϴ�Ӽ�����Ҫ�ɷ֣�
�dz���ϴ�Ӽ�����Ҫ�ɷ֣�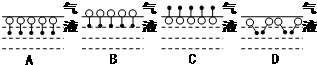
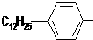 ��
��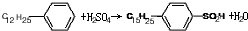 ����Ӧ����Ϊȡ����Ӧ��
����Ӧ����Ϊȡ����Ӧ������ ��1�����ӵļ��Ի���O�ˣ�Ӧ�ܽ���ˮ����ˮ��������������•�ˣ���Ӧ�ܽ���ˮ����ˮ������
��2��A�м��Ի��������У��ɼ��ٷ���֮��ij�����D�м��Ի���O�ˣ����⡢��������•�ˣ����ڵ����У���һ���̶���ʹ��ˮ����������ˮ�ĽӴ���
��3���������������ͻ���
��4��ʮ������������ᷴӦ���ɶ�ʮ����������
��5�����Ӵ�������Ҫ���˵�������ø�����ǵ������ڲ����˵����������ױ��ʣ�
��6���������������ˮ�帻Ӫ�������ƻ���ˮ��ԭ�е���̬ƽ�⣮
��� �⣺��1��ˮ����Ϊ���Է��ӣ����ӵļ��Ի���O�ˣ�Ӧ�ܽ���ˮ����ˮ��������������•�ˣ���Ӧ�ܽ���ˮ����ˮ�������ʴ�Ϊ��C��ˮ����Ϊ���Է��ӣ�������������ԭ�������ӵļ��Ի���O�ˣ�Ӧ�ܽ���ˮ����ˮ��������������•�ˣ���Ӧ�ܽ���ˮ����ˮ������
��2��A�м��Ի��������У��ɼ��ٷ���֮��ij�����D�м��Ի���O�ˣ����⡢��������•�ˣ����ڵ����У���һ���̶���ʹ��ˮ����������ˮ�ĽӴ���ʹ��ϵ������ͣ��ʴ�Ϊ��AD�� A�м��Ի��������У��ɼ��ٷ���֮��ij�����D�м��Ի���O�ˣ����⡢��������•�ˣ����ڵ����У���һ���̶���ʹ��ˮ����������ˮ�ĽӴ���ʹ��ϵ������ͣ�
��3���������������ͻ��� �����ͻ����ʴ�Ϊ��
�����ͻ����ʴ�Ϊ�� ��
��
��4��ʮ������������ᷴӦ���ɶ�ʮ����������ᣬ����ʽ��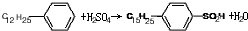 ������ȡ����Ӧ���ʴ�Ϊ��
������ȡ����Ӧ���ʴ�Ϊ��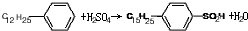 ��ȡ����Ӧ��
��ȡ����Ӧ��
��5��ø���Ӵ�������Ҫ���˵��������¶ȡ����ȵȣ���ø�����ǵ������ڲ����˵����������ױ��ʣ�����£����ʴ�Ϊ��ø���Ӵ�������Ҫ���˵��������¶ȡ����ȵȣ���ø�����ǵ������ڲ����˵�����������¡�ˮ�⣩�����ױ��ʣ�
��6���������������ˮ�帻Ӫ�������ƻ���ˮ��ԭ�е���̬ƽ�⣬����������滷������˺ܴ��Ӱ�죬�ʴ�Ϊ���������������ˮ�帻Ӫ�������ƻ���ˮ��ԭ�е���̬ƽ�⣬����������滷������˺ܴ��Ӱ�죮
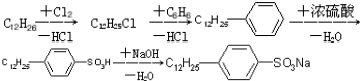
���� ���⿼���ǶԻ�ѧ�뼼���Ŀ��飬�漰�ϳ�ϴ�Ӽ�����֬�����ʡ�ø�����ʡ���������ȣ��Ƚϻ���������ϳ�ϴ�Ӽ��б�����Լ������ͻ��ź���ˮ���������ֹ��ɣ������ڻ���֪ʶ�Ĺ��̣�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 L��Һ�к�Ba2+��N${O}_{3}^{-}$��������0.6NA | |
| B�� | 500 mL��Һ��Ba2+��Ũ����0.2 mol•L-1 | |
| C�� | 500 mL��Һ��N${O}_{3}^{-}$��Ũ����0.2 mol•L-1 | |
| D�� | 1 L��Һ�к���0.4NA��N${O}_{3}^{-}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������չ�������磬��������������� | |
| B�� | ���ݶ���װ̫������ˮ��Ϊ�����ṩ��������ˮ | |
| C�� | Ϊ�˼��ٶ�������Ͷ����������ŷţ���ҵ�����ŷŵ�����֮ǰ������մ��� | |
| D�� | ���Ӳݡ������������л����������������з��Ͳ�������������ͥȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
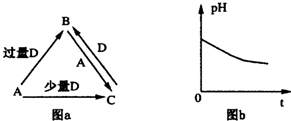
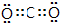 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������SO2����ͨ��NaClO��Һ�У�SO2+2ClO-+H2O�TSO32-+2HClO | |
| B�� | �����������Һ�м�������������Һ��pH=7 Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O | |
| C�� | Na2SO3��Һʹ����KMnO4��Һ��ɫ��5SO32-+6H++2MnO4-�T5SO42-+2Mn2++3H2O | |
| D�� | ��NaHCO3 ��Һ�е��������Ba��OH��2��Һ��HCO3-+Ba2++OH-�TH2O+BaCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�11.2 L���к��е�̼̼˫����ĿΪ1.5NA | |
| B�� | 25��ʱ��1.0 L pH=12��Na2 CO3��Һ�к��е�������������0.01NA | |
| C�� | ��״���£�2.8 g��N2��CO��ɵĻ�������к��е�ԭ����Ϊ0.1NA | |
| D�� | �ڷ�Ӧ��Cu2S+O2$\frac{\underline{\;����\;}}{\;}$2Cu+SO2�У�����1 mol Cu��ת�Ƶĵ�����Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��HCO3-��MnO4- | B�� | Al3+��Mg2+��HCO3- | ||
| C�� | Al3+��Mg2+��SO42- | D�� | Al3+��Mg2+��NH4+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��������� | ����1 | ����2 | |
| A | ����狀���� | ��ˮ�ܽ� | ����ʯ�� |
| B | һ����̼�Ͷ�����̼ | ����ζ | ͨ�����ȵ�����ͭ |
| C | ʳ��ˮ������ˮ | ��pH | �����ᾧ |
| D | ��ƽ�ͼٻƽ� ��ͭ���Ͻ� | ����ɫ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com