
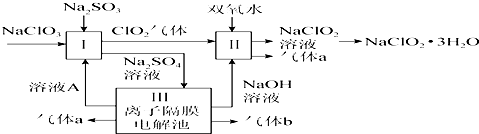
·ÖĪö øł¾ŻĢāÖŠĮ÷³Ģ£¬¢ńÖŠNaClO3ŌŚĖįŠŌĢõ¼žĻĀ±»ŃĒĮņĖįøłĄė×Ó»¹Ō³ÉClO2£¬·“Ó¦¢ņClO2ŌŚ¼īŠŌĢõ¼žĻĀ±»Ė«ŃõĖ®»¹Ō³ÉNaClO2£¬NaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬ĶعżÕō·¢ÅØĖõ£¬ĄäČ“½į¾§£¬¹żĀĖĻ“µÓµĆµ½¾§ĢåNaClO2£®
£Ø1£©µē½āĮņĖįÄʵƵ½µÄŹĒĒāĘųŗĶŃõĘų£¬µē½āĮņĖįÄĘČÜŅŗæÉŅŌŌŚĮ½øöµē¼«ÉĻµĆµ½ĒāŃõ»ÆÄĘŗĶĮņĖį£»
£Ø2£©¢ńÖŠNaClO3ŌŚĖįŠŌĢõ¼žĻĀ±»ŃĒĮņĖįøłĄė×Ó»¹Ō³ÉClO2£¬¾Ż“ĖČ·¶Ø·“Ó¦ÖŠµÄŃõ»Æ¼ĮŗĶ»¹Ō¼ĮŅ»Ęš¶žÕßµÄĮæÖ®±Č£»
£Ø3£©·“Ó¦¢ņClO2ŌŚ¼īŠŌĢõ¼žĻĀ±»Ė«ŃõĖ®»¹Ō³ÉNaClO2£»
£Ø4£©“ÓČÜŅŗÖŠµĆµ½ŗ¬½į¾§Ė®µÄ¾§Ģ壬ֻÄܲÉČ”Õō·¢”¢ÅØĖõ”¢ĄäČ“½į¾§·½·Ø£¬Ķعż¹żĀĖµĆµ½“Ö¾§Ģ壬µĆµ½µÄ“Ö¾§Ģå¾¹żÖŲ½į¾§æɵƵ½“æ¶Čøüøߵľ§Ģ壻
£Ø5£©øł¾ŻµāÓöµ½µķ·Ū±äĄ¶É«½įŗĻ·“Ó¦µÄŌĄķÅŠ¶Ļ·“Ó¦ÖÕµć£»Č”ÉĻŹöŗĻ³É²śĘ·10gČÜÓŚĖ®Åä³É500mLČÜŅŗ£¬Č”³ö10mLČÜŅŗӌ׶ŠĪĘæÖŠ£¬ŌŁ¼ÓČė×ćĮæĖį»ÆµÄKIČÜŅŗ£¬·¢ÉśClO2-+4I-+4H+=Cl-+2I2+2H2O£¬³ä·Ö·“Ó¦ŗó¼ÓČė2”«3µĪµķ·ŪČÜŅŗ£¬ČÜŅŗ±äĄ¶£¬ÓĆ0.264mol/L Na2S2O3±ź×¼ŅŗµĪ¶Ø£¬·¢Éś£ŗ2Na2S2O3+I2ØTNa2S4O6+2NaI£¬Ą¶É«±äĪŖĪŽÉ«£¬æɵƷ“Ó¦µÄ¹ŲĻµŹ½ĪŖ£ŗClO2-”«2I2”«4Na2S2O3£¬¾Ż“Ė¼ĘĖć¼“æÉ£®
½ā“š ½ā£ŗøł¾ŻĢāÖŠĮ÷³Ģ£¬¢ńÖŠNaClO3ŌŚĖįŠŌĢõ¼žĻĀ±»ŃĒĮņĖįøłĄė×Ó»¹Ō³ÉClO2£¬·“Ó¦¢ņClO2ŌŚ¼īŠŌĢõ¼žĻĀ±»Ė«ŃõĖ®»¹Ō³ÉNaClO2£¬NaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬ĶعżÕō·¢ÅØĖõ£¬ĄäČ“½į¾§£¬¹żĀĖĻ“µÓµĆµ½¾§ĢåNaClO2£®
£Ø1£©µē½āĮņĖįÄʵƵ½µÄŹĒĒāĘųŗĶŃõĘų£¬·“Ó¦¢ņµÄ»Æѧ·½³ĢŹ½ĪŖ2NaOH+2ClO2+H2O2ØT2NaClO2+2H2O+O2£¬ĖłŅŌaŹĒŃõĘų£¬bŹĒĒāĘų£¬µē½āĮņĖįÄĘČÜŅŗæÉŅŌŌŚĮ½øöµē¼«ÉĻµĆµ½ĒāŃõ»ÆÄĘŗĶĮņĖį£¬ĖłŅŌČÜŅŗAµÄĆū³ĘŹĒĮņĖį£¬¹Ź“š°øĪŖ£ŗO2£»ĮņĖį£»
£Ø2£©¢ńÖŠNaClO3ŌŚĖįŠŌĢõ¼žĻĀ±»ŃĒĮņĖįøłĄė×Ó»¹Ō³ÉClO2£¬·“Ó¦IµÄĄė×Ó·½³ĢŹ½ĪŖ2ClO3-+SO32-+2H+$\frac{\underline{\;”÷\;}}{\;}$2ClO2”ü+SO42-+H2O£¬ĘäÖŠŃõ»Æ¼ĮŹĒClO3-£¬»¹Ō¼ĮŹĒSO32-£¬¶žÕßµÄĪļÖŹµÄĮæÖ®±ČĪŖŹĒ2£ŗ1£¬¹Ź“š°øĪŖ£ŗ2ClO3-+SO32-+2H+$\frac{\underline{\;”÷\;}}{\;}$2ClO2”ü+SO42-+H2O£»2£ŗ1£»
£Ø3£©·“Ó¦¢ņClO2ŌŚ¼īŠŌĢõ¼žĻĀ±»Ė«ŃõĖ®»¹Ō³ÉNaClO2£¬»Æѧ·½³ĢŹ½ĪŖ2NaOH+2ClO2+H2O2ØT2NaClO2+2H2O+O2£¬ĪŖĮĖ·ĄÖ¹H2O2·Ö½ā£¬·“Ó¦¹ż³ĢÖŠæŲÖĘĪĀ¶Č²»øßÓŚ20”ę£¬¹Ź“š°øĪŖ£ŗ£©2NaOH+2ClO2+H2O2ØT2NaClO2+2H2O+O2£»·ĄÖ¹H2O2·Ö½ā£»
£Ø4£©“ÓČÜŅŗÖŠµĆµ½ŗ¬½į¾§Ė®µÄ¾§Ģ壬ֻÄܲÉČ”Õō·¢”¢ÅØĖõ”¢ĄäČ“½į¾§·½·Ø£¬Ķعż¹żĀĖµĆµ½“Ö¾§Ģ壬µĆµ½µÄ“Ö¾§Ģå¾¹żÖŲ½į¾§æɵƵ½“æ¶Čøüøߵľ§Ģ壻
¹Ź“š°øĪŖ£ŗB”¢E”¢D£»ÖŲ½į¾§£»
£Ø5£©µ±µĪČė×īŗóŅ»µĪNa2S2O3±ź×¼ŅŗŹ±£¬×¶ŠĪĘæÖŠČÜŅŗÓÉĄ¶É«±äĪŖĪŽÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»·¢Éś±ä»Æ£¬ĖµĆ÷µĪ¶Ø“ļÖÕµć£»
Č”ÉĻŹöŗĻ³É²śĘ·10gČÜÓŚĖ®Åä³É500mLČÜŅŗ£¬Č”³ö10mLČÜŅŗӌ׶ŠĪĘæÖŠ£¬ŌŁ¼ÓČė×ćĮæĖį»ÆµÄKIČÜŅŗ£¬·¢ÉśClO2-+4I-+4H+=Cl-+2I2+2H2O£¬³ä·Ö·“Ó¦ŗó¼ÓČė2”«3µĪµķ·ŪČÜŅŗ£¬ČÜŅŗ±äĄ¶£¬ÓĆ0.264mol/L Na2S2O3±ź×¼ŅŗµĪ¶Ø£¬·¢Éś£ŗ2Na2S2O3+I2ØTNa2S4O6+2NaI£¬Ą¶É«±äĪŖĪŽÉ«£¬
æɵƷ“Ó¦µÄ¹ŲĻµŹ½ĪŖ£ŗClO2-”«2I2”«4Na2S2O3£¬ÓÖn£ØNa2S2O3£©=0.264mol/L”Į0.02L=0.00528mol£¬
ŌņClO2-”«2I2”«4Na2S2O3
1 4
n£ØClO2-£© 0.00528mol
n£ØClO2-£©=$\frac{0.00528mol}{4}$=0.00132mol£¬
ĖłŅŌ500mlČÜŅŗÖŠŗ¬ÓŠ£ŗn£ØNaClO2£©=0.00132mol”Į50=0.066mol£¬
Ōņ10gŗĻ³É²śĘ·ÖŠŗ¬ÓŠ£ŗm£ØNaClO2•3H2O£©=0.066mol”Į144.5g/mol=9.537g£¬
Ōņ¦Ų£ØNaClO2•3H2O£©=$\frac{9.537g}{10g}$”Į100%=95.37%£®
¹Ź“š°øĪŖ£ŗÓÉĄ¶É«±äĪŖĪŽÉ«£»95.37%£®
µćĘĄ ±¾Ģāæ¼²é½ĻĪŖ×ŪŗĻ£¬Éę¼°ĪļÖŹµÄ¼ģŃ锢³żŌÓ”¢ÖʱøŅŌ¼°ŗ¬ĮæµÄ²ā¶Ø£¬ĢāÄæÄŃ¶Č½Ļ“󣬱¾Ģā£Ø5£©ĪŖŅדķµć£¬×¢Ņā°ŃĪÕÓŠ¹Ų·“Ó¦·½³ĢŹ½µÄŹéŠ“£®


 Źī¼ŁĻĪ½Ó½Ģ²ÄĘŚÄ©Źī¼ŁŌ¤Ļ°Īäŗŗ³ö°ęÉēĻµĮŠ“š°ø
Źī¼ŁĻĪ½Ó½Ģ²ÄĘŚÄ©Źī¼ŁŌ¤Ļ°Īäŗŗ³ö°ęÉēĻµĮŠ“š°ø ¼ŁĘŚ×÷ŅµŹī¼Ł³É³¤ĄÖŌ°ŠĀ½®ĒąÉŁÄź³ö°ęÉēĻµĮŠ“š°ø
¼ŁĘŚ×÷ŅµŹī¼Ł³É³¤ĄÖŌ°ŠĀ½®ĒąÉŁÄź³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| t/s | 0 | 500 | 1 000 | 1 500 |
| c£ØA£©/£Ømol•L-1£© | 6.00 | 3.50 | 3.00 | 3.00 |
| A£® | 500sÄŚAµÄ·Ö½āĖŁĀŹĪŖ3.0”Į10-3 mol•L-1•s-1 | |
| B£® | 1000sŹ±AµÄ×Ŗ»ÆĀŹĪŖ50%£¬T1ĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżĪŖK1=0.75 | |
| C£® | T1ĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżĪŖK1£¬T2ĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżĪŖK2£¬ČōK1£¾K2£¬ŌņT1£¾T2 | |
| D£® | Ę½ŗāŹ±£¬ĘäĖūĢõ¼ž²»±ä£¬ŌŁ¼ÓČėŅ»¶ØĮæµÄA£¬“ļŠĀĘ½ŗāŗóKÖµŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
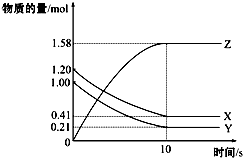 Ņ»¶ØĪĀ¶ČĻĀ£¬ŌŚ2LµÄĆܱÕČŻĘ÷ÖŠ£¬X”¢Y”¢ZČżÖÖĘųĢåµÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗĻĀĮŠĆčŹöÕżČ·µÄŹĒ£Ø””””£©
Ņ»¶ØĪĀ¶ČĻĀ£¬ŌŚ2LµÄĆܱÕČŻĘ÷ÖŠ£¬X”¢Y”¢ZČżÖÖĘųĢåµÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£ŗĻĀĮŠĆčŹöÕżČ·µÄŹĒ£Ø””””£©| A£® | ·“Ó¦æŖŹ¼µ½10s£¬ÓĆZ±ķŹ¾µÄ·“Ó¦ĖŁĀŹĪŖ0.158 mol/£ØL•s£© | |
| B£® | ·“Ó¦æŖŹ¼µ½10s£¬XµÄĪļÖŹµÄĮæÅØ¶Č¼õÉŁĮĖ0.79 mol/L | |
| C£® | ·“Ó¦æŖŹ¼µ½10sŹ±£¬YµÄ×Ŗ»ÆĀŹĪŖ79.0% | |
| D£® | ŌŚĒ°10sÄŚÓĆX”¢Y”¢Z±ķŹ¾µÄ·“Ó¦ĖŁĀŹŹżÖµĻąµČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | v£ØC£©=0.5mol/£ØL•min£© | |
| B£® | x=3 | |
| C£® | BµÄ×Ŗ»ÆĀŹĪŖ25% | |
| D£® | ČōŹ¹ÓĆ“ß»Æ¼ĮæÉŅŌĖõ¶Ģ“ļµ½Ę½ŗāµÄŹ±¼ä£¬µ«A×Ŗ»ÆĀŹ²»±ä |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
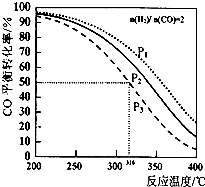 ŅŌ$\frac{n£Ø{H}_{2}£©}{n£ØCO£©}$=2 ĶØČėILµÄ·“Ó¦Ę÷ÖŠ£¬Ņ»¶ØĢõ¼žT ·¢Éś·“Ó¦£ŗ4H2£Øg£©+2CO£Øg£©?CH3OCH3£Øg£©+H2O £Øg£©”÷H£¬ĘäÖŠCOµÄĘ½ŗā×Ŗ»ÆĀŹĖęĪĀ¶Č”¢Ń¹Ēæ±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ŅŌ$\frac{n£Ø{H}_{2}£©}{n£ØCO£©}$=2 ĶØČėILµÄ·“Ó¦Ę÷ÖŠ£¬Ņ»¶ØĢõ¼žT ·¢Éś·“Ó¦£ŗ4H2£Øg£©+2CO£Øg£©?CH3OCH3£Øg£©+H2O £Øg£©”÷H£¬ĘäÖŠCOµÄĘ½ŗā×Ŗ»ÆĀŹĖęĪĀ¶Č”¢Ń¹Ēæ±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | øĆ·“Ó¦µÄ”÷H£¾O | |
| B£® | P1£¼P2£¼P3 | |
| C£® | ČōŌŚP3ŗĶ316”ꏱ·“Ó¦“ļµ½Ę½ŗā£¬H2µÄ×Ŗ»ÆĀŹµČÓŚ50% | |
| D£® | ČōŌŚP1ŗĶ200”ꏱ£¬·“Ó¦“ļĘ½ŗāŗó±£³ÖĪĀ¶ČŗĶŃ¹Ēæ²»±ä£¬ŌŁ³äČė2 mol H2ŗĶ1molCO£¬ŌņĘ½ŗāŹ±¶ž¼×ĆѵÄĢå»ż·ÖŹżŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µ±b£¾2aŹ±£¬·¢ÉśµÄĄė×Ó·“Ó¦ĪŖ£ŗCO32-+2H+=H2O+CO2”ü | |
| B£® | µ±b£¼aŹ±£¬·¢ÉśµÄĄė×Ó·“Ó¦ĪŖ£ŗCO32-+H+=HCO3- | |
| C£® | µ±4a=3bŹ±£¬·¢ÉśµÄĄė×Ó·“Ó¦ĪŖ£ŗ3CO32-+4H+=2HCO3-+CO2”ü+H2O | |
| D£® | µ±a£¼b£¼2aŹ±£¬·“Ӧɜ³ÉµÄHCO3-ÓėCO2µÄĪļÖŹµÄĮæÖ®±ČĪŖ£Øb-a£©£ŗ£Ø2b-a£© |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com