
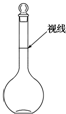
 ʵ��������500mL 0.2mol•L-1��Na2SO4��Һ��ʵ����������У�
ʵ��������500mL 0.2mol•L-1��Na2SO4��Һ��ʵ����������У����� ��1������������Һ��ʵ��������̽��в������������
��2������������Һ��ʵ���������ѡ������������
��3�������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������500ml0.2mol/L��Na2SO4��Һ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������������ƽ��������ҩ��ȡҩƷ�����ձ����ܽ⣨������Ͳ��ˮ�����ò��������裬�����ܽ⣬�ָ������º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���˳��ΪABDCE��
�ʴ�Ϊ��ABDCE��
��2������500ml0.2mol/L��Na2SO4��Һ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ������õ��������У�������ƽ���ձ�������������Ͳ��500mL����ƿ����ͷ�ιܣ�
��ȱ�ٵ������У���Ͳ��500ml����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����Ͳ��500ml����ƿ����ͷ�ιܣ�
��3��a��ijͬѧ�۲�Һ��������ͼ��ʾ��������Һ�棬����Һ���ƫС��Ũ��ƫ�ߣ���a��ȷ��
b��û���������IJ�������D���������ʵ���ʧ����Ũ��ƫ�ͣ���b����
c��������ˮʱ�����������˿̶��ߣ�����Һ���ƫ��Ũ��ƫС����c����
d��������մ�����ʣ������������ƫ��������ҩƷ������ƫ����Ũ��ƫ�ߣ���d��ȷ��
e������ƿʹ��ǰ�ڱ�մ��ˮ�飬��Ũ����Ӱ�죬��e����
��ѡad��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ���ƹ����ǽ���ؼ���ע����������������Ŀ�ѶȲ���


 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X����ijЩ�ǽ���Ԫ�ػ��� | |
| B�� | X����Ϊ����Ԫ�� | |
| C�� | Xԭ�ӵ������������ͺ˵�����϶�Ϊ���� | |
| D�� | X�����γɻ�ѧʽΪKXO3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ȼ��þ���������CO2����ļ���ƿ�У�2Mg+CO2�T��ȼ 2MgO+C | |
| B�� | ����ʯ���ڴ��CO32-+2CH3COOH�T2CH3COO-+CO2��+H2O | |
| C�� | ��NH4HSO4ϡ��Һ����μ���Ba��OH��2ϡ��Һ��SO42-�պó�����ȫ��Ba2++2OH-+NH4++H++SO42-�TBaSO4��+NH3•H2O+H2O | |
| D�� | �����KI��Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ�����Һ������4H++4I-+O2�T2I2+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Zn��s��+H2SO4��aq���TZnSO4��aq��+H2����g����H��0 | B�� | 2CO��g��+O2��g���T2CO2��g����H��0 | ||
| C�� | C��s��+CO2��g���T2 CO��g����H��0 | D�� | H+��aq��+OH-��aq���TH2O ��l����H��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��С�������ݻ� | B�� | ʹ�ô��� | C�� | ��ѹ�³���He | D�� | �����³���Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Na2CO3+SiO2$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2����֪������H2SiO3��H2CO3 | |
| B�� | �������Ҫ�ܷ����������IJ����Լ�ƿ�� | |
| C�� | ���������Һ�м������������ɫ����������ʱ�����ܽ� | |
| D�� | ��������������������������Ϊ�ڻ�NaOH�����װ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H6 | B�� | C4H10 | C�� | C5H12 | D�� | C7H16 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| T/�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com