

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��C��ʯī�� | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | ����������Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
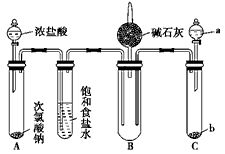
 ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ú�ʵ�鷽�����г�������ʡ�ԣ�����ݴ���ش���Ӧ���⣮
ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ú�ʵ�鷽�����г�������ʡ�ԣ�����ݴ���ش���Ӧ���⣮| �� |
| ||
| ||
| 3b |
| 11a |
| 3b |
| 11a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
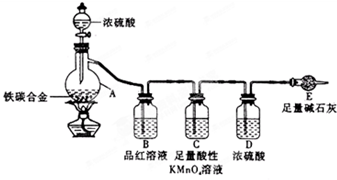
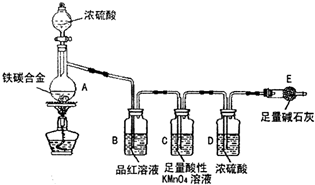 ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ𣨿�������̼���ֵ��ʵĻ���������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��װ�ã��г�������ʡ�ԣ��ͷ�������ʵ�飮
ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ𣨿�������̼���ֵ��ʵĻ���������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��װ�ã��г�������ʡ�ԣ��ͷ�������ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
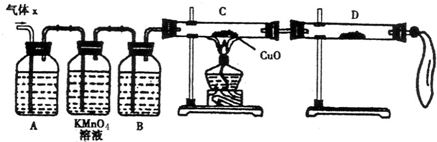
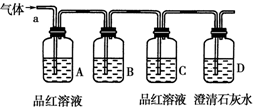 ij��ѧ��ȤС��Ϊ��̽��������ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֳ���ʯ��ˮ����ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮
ij��ѧ��ȤС��Ϊ��̽��������ij�ǽ����������γɵ�δ֪����ijɷ֣���С���Ա������ͨ�����ʯ��ˮ�����ֳ���ʯ��ˮ����ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������룮�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com