
”¾ĢāÄæ”æ(1)ÓŠĮ½ÖÖ»īŠŌ·“Ó¦ÖŠ¼äĢåĄė×Ó£¬ĖüĆĒµÄĄė×ÓÖŠ¾łŗ¬ÓŠ1øöĢ¼Ō×ÓŗĶ3øöĒāŌ×Ó”£ĒėŅĄ¾ŻĻĀĆęøų³öµÄÕāĮ½ÖÖĪ¢Į£µÄĒņ¹÷Ä£ŠĶ£¬Š“³öĻąÓ¦µÄ»ÆѧŹ½£ŗ
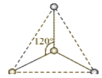 ___________£»
___________£» ______________”£
______________ӣ
(2)°“ŅŖĒ󊓳öµŚ¶žÖÜĘŚ·Ē½šŹōŌŖĖŲ¹¹³ÉµÄÖŠŠŌ·Ö×ӵĻÆѧŹ½”£
Ę½ĆęČż½ĒŠĪ·Ö×Ó___________£¬Čż½Ē׶ŠĪ·Ö×Ó____________£¬ÕżĖÄĆęĢåŠĪ·Ö×Ó_____________”£
(3)Š“³öSO3µÄ³£¼ūµÄµČµē×ÓĢåµÄ»ÆѧŹ½£ŗŅ»¼ŪŅõĄė×Ó____________ (Š“³öŅ»ÖÖ£¬ĻĀĶ¬)£¬¶ž¼ŪŅõĄė×Ó____________£¬ĖüĆĒµÄÖŠŠÄŌ×Ó²ÉÓƵÄŌӻƷ½Ź½¶¼ŹĒ____________”£
”¾“š°ø”æCH3+ CH3- BF3 NF3 CF4 NO3- CO32- sp2
”¾½āĪö”æ
(1)øł¾Ż·Ö×ÓµÄæռ乹ŠĶ”¢ŌÓ»ÆĄąŠĶŅŌ¼°¼Ū²ćµē×Ó¶ŌŹż½įŗĻĮ£×ÓÖŠ¾łŗ¬ÓŠ1øöĢ¼Ō×ÓŗĶ3øöĒāŌ×ÓĄ“·ÖĪöÅŠ¶Ļ£»
(2)ÓɵŚ¶žÖÜĘŚ·Ē½šŹōŌŖĖŲ¹¹³ÉµÄÖŠŠŌ·Ö×Ó£¬µŚ¶žÖÜĘŚŌŖĖŲĪŖÖŠŠÄŌ×Ó£¬Čē¹ūŹĒĘ½ĆęŠĪ·Ö×Ó£¬ŌņĶعżsp2ŌӻƊĪ³ÉÖŠŠŌ·Ö×Ó£»Čē¹ūŹĒČż½Ē׶ŠĶ·Ö×Ó£¬ŌņĶعżsp3ŌӻƊĪ³ÉÖŠŠŌ·Ö×Ó£¬ĒŅ¼Ū²ćµē×Ó¶ŌøöŹżŹĒ4£¬ŗ¬ÓŠŅ»øö¹Āµē×Ó¶Ō£»Čē¹ūŹĒÕżĖÄĆęĢå½į¹¹£¬ŌņøĆ·Ö×ӵļŪ²ćµē×Ó¶ŌøöŹżŹĒ4ĒŅ²»ŗ¬¹Āµē×Ó¶Ō£¬¾Ż“Ė·ÖĪö½ā“š£»
(3)µČµē×ÓĢåŹĒÖø¾ßÓŠĻąĶ¬¼Ūµē×Ó×ÜŹżŗĶŌ×Ó×ÜŹżµÄ·Ö×Ó»ņĄė×Ó£»øł¾Ż¼Ūµē×Ó¶Ō»„³āĄķĀŪČ·¶ØŌ×ÓµÄŌӻƷ½Ź½£¬¼Ū²ćµē×Ó¶ŌøöŹż=¦Ņ¼üøöŹż+¹Āµē×Ó¶ŌøöŹż¼ĘĖćÅŠ¶Ļ”£
(1)µŚŅ»ÖÖĪ¢Į£µÄæÕ¼ä½į¹¹ĪŖĘ½ĆęČż½ĒŠĪ£¬ŌņĢ¼Ō×ÓĪŖsp2Ōӻƣ¬ÖŠŠÄĢ¼Ō×ÓĪŽ¹Āµē×Ó¶Ō£¬Ņņ“Ė¼Ū²ćµē×Ó¶ŌŹż3£¬»ÆѧŹ½ĪŖCH3+£¬µŚ¶žÖÖĪ¢Į£µÄæÕ¼ä½į¹¹ĪŖČż½Ē׶ŠĪ£¬ŌņĢ¼Ō×ÓĪŖsp3Ōӻƣ¬ÖŠŠÄĢ¼Ō×ÓÓŠ1øö¹Āµē×Ó¶Ō£¬Ņņ“Ė¼Ū²ćµē×Ó¶ŌŹż4£¬»ÆѧŹ½CH3-£¬¹Ź“š°øĪŖ£ŗCH3+£»CH3-£»
(2)ÓɵŚ¶žÖÜĘŚ·Ē½šŹōŌŖĖŲ¹¹³ÉµÄÖŠŠŌ·Ö×Ó£¬µŚ¶žÖÜĘŚŌŖĖŲĪŖÖŠŠÄŌ×Ó£¬Ķعżsp2ŌӻƊĪ³ÉÖŠŠŌ·Ö×Ó£¬ŹĒĘ½ĆęŠĪ·Ö×Ó£¬øĆĄąŠĶ·Ö×ÓÓŠBF3£»µŚ¶žÖÜĘŚŌŖĖŲĪŖÖŠŠÄŌ×Ó£¬Ķعżsp3ŌӻƊĪ³ÉÖŠŠŌ·Ö×Ó£¬Čē¹ūŹĒČż½Ē׶ŠĶ·Ö×Ó£¬ŌņøĆ·Ö×ÓÖŠ¼Ū²ćµē×Ó¶ŌøöŹżŹĒ4ĒŅŗ¬ÓŠŅ»øö¹Āµē×Ó¶Ō£¬øĆĄąŠĶ·Ö×ÓÓŠNF3£»Čē¹ūøĆ·Ö×ÓĪŖÕżĖÄĆęĢå½į¹¹£¬ŌņøĆ·Ö×ӵļŪ²ćµē×Ó¶ŌøöŹżŹĒ4ĒŅ²»ŗ¬¹Āµē×Ó¶Ō£¬øĆĄąŠĶ·Ö×ÓÓŠCF4£¬¹Ź“š°øĪŖ£ŗBF3£»NF3£»CF4£»
(3)SO3µÄŌ×ÓŹżĪŖ4£¬¼Ūµē×ÓŹżĪŖ24£¬ÓėSO3»„ĪŖµČµē×ÓĢåµÄĪŖNO3-»ņCO32-”¢BF3»ņCOCl2µČ£»NO3-ÖŠNŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬Ć»ÓŠ¹Āµē×Ó¶Ō£¬ŌÓ»ÆĄąŠĶĪŖsp2£¬Ģ¼ĖįøłĄė×ÓÖŠ¼Ū²ćµē×Ó¶ŌøöŹż=¦Ņ¼üøöŹż+¹Āµē×Ó¶ŌøöŹż=3+![]() (4+2-3”Į2)=3£¬ĖłŅŌŌ×ÓŌӻƷ½Ź½ŹĒsp2£¬SO3·Ö×ÓÖŠSŌ×ӵļŪ²ćµē×Ó¶ŌøöŹż=¦Ņ¼üøöŹż+¹Āµē×Ó¶ŌøöŹż=3+
(4+2-3”Į2)=3£¬ĖłŅŌŌ×ÓŌӻƷ½Ź½ŹĒsp2£¬SO3·Ö×ÓÖŠSŌ×ӵļŪ²ćµē×Ó¶ŌøöŹż=¦Ņ¼üøöŹż+¹Āµē×Ó¶ŌøöŹż=3+![]() =3£¬ŌÓ»ÆĄąŠĶĪŖsp2£¬¹Ź“š°øĪŖ£ŗNO3-£»CO32-£»sp2”£
=3£¬ŌÓ»ÆĄąŠĶĪŖsp2£¬¹Ź“š°øĪŖ£ŗNO3-£»CO32-£»sp2”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æCuSO4ŹĒŅ»ÖÖÖŲŅŖ»Æ¹¤ŌĮĻ£¬ĘäÖʱøŗĶÓŠ¹ŲŠŌÖŹČēĶ¼ĖłŹ¾”£
£Ø1£©ĻÖŅŖÓĆČēĶ¼ĖłŹ¾µÄÅØĮņĖįĄ“ÅäÖĘ²½Öč¢ŁÖŠĖłŠčŅŖµÄ1mol/LµÄĻ”ĮņĖį480ml£¬ŠčŅŖÓĆÕāÖÖÅØĮņĖįµÄĢå»żĪŖ______ml”£
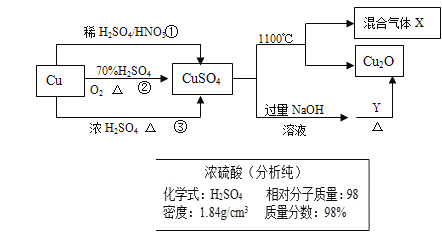
£Ø2£©ÅäÖĘøĆĻ”ĮņĖįĖłÓƵ½µÄ²£Į§ŅĒĘ÷³ż²£Į§°ō”¢ĮæĶ²”¢ÉÕ±Ķā»¹ÓŠ_______”¢__________”£
£Ø3£©ĻĀĮŠ²Ł×÷»įŹ¹ĖłÅäČÜŅŗÅضČĘ«µĶµÄŹĒ _________”£
A. ĮņĖį×ŖŅĘÖĮČŻĮæĘæŗó£¬Ć»ÓŠĻ“µÓÉÕ±
B. Ī“ĄäČ“ÖĮŹŅĪĀ¾Ķ×ŖŅĘÖĮČŻĮæĘæ
C. ČŻĮæĘæÖŠ“ęŌŚÉŁĮæÕōĮóĖ®
D. ¶ØČŻŹ±ø©ŹÓæĢ¶Č E.ĮæČ”ÅØĮņĖįŹ±ĮæĶ²ÄŚÓŠÉŁĮæÕōĮóĖ®
£Ø4£©ÖĘČ”ĮņĖįĶµÄĶ¾¾¶¢Ł¢Ś¢ŪÖŠ£¬Ķ¾¾¶_________ÄÜøüŗƵŲĢåĻÖĀĢÉ«»ÆѧµÄĖ¼Ļė”£
£Ø5£©ÅäÖĘ1000ml 0.1mol/LµÄĮņĖįĶČÜŅŗ£¬ŠčÓĆĶŠÅĢĢģĘ½³ĘČ”________gµØ·Æ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĀČĘųŌŚ¹¤Å©ŅµÉś²śŌŚÓ¦ÓĆ·Ē³£¹ć·ŗ”£ĻĀĶ¼ŹĒŹµŃéŹŅÖʱøĀČĘų²¢½ųŠŠŅ»ĻµĮŠĻą¹ŲŹµŃéµÄ×°ÖĆ”£
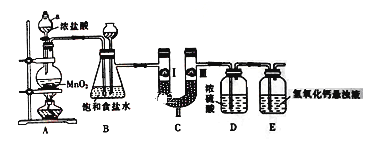
(1) ŅĒĘ÷aµÄĆū³ĘĪŖ_______________”£
(2) ×°ÖĆBÖŠ±„ŗĶŹ³ŃĪĖ®µÄ×÷ÓĆŹĒ_________________£»
(3) Š“³öA×°ÖĆÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½______________________
(4) ×°ÖĆCµÄ×÷ÓĆŹĒŃéÖ¤ĀČĘųŹĒ·ń¾ßÓŠĘư׊Ō£¬ĪŖ“Ė×°ÖĆCÖŠI”¢II”¢III“¦ŅĄ“Ī·ÅČė__________(Ģī×ÖÄø)”£
Ń”Ļī | a | b | c |
I | ŹŖČóµÄÓŠÉ«²¼Ģõ | ŹŖČóµÄÓŠÉ«²¼Ģõ | ŹŖČóµÄÓŠÉ«²¼Ģõ |
II | ¼īŹÆ»Ņ | ÅØĮņĖį | ĪŽĖ®ĀČ»ÆøĘ |
III | ŹŖČóµÄÓŠÉ«²¼Ģõ | øÉŌļµÄÓŠÉ«²¼Ģõ | øÉŌļµÄÓŠÉ«²¼Ģõ |
(5) ×°ÖĆEÖŠĒāŃõ»ÆøĘŠü×ĒŅŗµÄ×÷ÓĆŹĒ___________________£¬Ķ¬Ź±øĆ×°ÖĆ¹¤ŅµÉĻæÉÓĆĄ“ÖĘČ”ĘÆ°×·Ū£¬ĒėŠ“³öĻąÓ¦·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ___________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĶ¼ĪŖCaF2”¢H3BO3(²ćד½į¹¹£¬²ćÄŚµÄH3BO3·Ö×ÓĶعżĒā¼ü½įŗĻ)”¢½šŹōĶČżÖÖ¾§ĢåµÄ½į¹¹Ź¾ŅāĶ¼£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
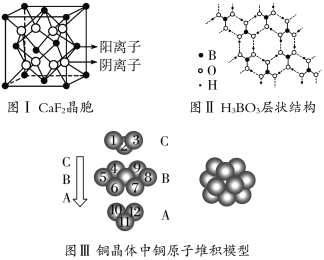
(1)Ķ¼¢ńĖłŹ¾µÄCaF2¾§ĢåÖŠÓėCa2£«×ī½üĒŅµČ¾ąĄėµÄF£ŹżĪŖ________£¬Ķ¼¢óÖŠĪ“±źŗŵÄĶŌ×ÓŠĪ³É¾§ĢåŗóÖÜĪ§×ī½ōĮŚµÄĶŌ×ÓŹżĪŖ________”£
(2)Ķ¼¢ņĖłŹ¾µÄĪļÖŹ½į¹¹ÖŠ×īĶāÄܲćŅŃ“ļ8µē×Ó½į¹¹µÄŌ×ÓŹĒ________£¬H3BO3¾§ĢåÖŠBŌ×ÓøöŹżÓė¼«ŠŌ¼üøöŹż±ČĪŖ________”£
(3)ČżÖÖ¾§ĢåÖŠČŪµć×īµĶµÄŹĒ________£¬Ę侧ĢåŹÜČČČŪ»ÆŹ±£¬æĖ·žµÄĪ¢Į£Ö®¼äµÄĻą»„×÷ÓĆĪŖ________________________________________________________________”£
(4)½įŗĻCaF2¾§ĢåµÄ¾§°ūŹ¾ŅāĶ¼£¬ŅŃÖŖ£¬Į½øö¾ąĄė×ī½üµÄCa2£«ŗĖ¼ä¾ąĄėĪŖa”Į10£8 cm£¬¼ĘĖćCaF2¾§ĢåµÄĆܶČĪŖ________g”¤cm£3”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ·Ö×ÓÖŠ£¬ÖŠŠÄŌ×ÓµÄŌӻƹģµĄĄąŠĶĻąĶ¬µÄŹĒ
A. CO2ÓėSO2B. CH4ÓėNH3
C. H2O2ÓėC2H4D. C2H4ÓėN2H4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æX”¢Y”¢Z”¢Q”¢EĪåÖÖŌŖĖŲÖŠ£¬XŌ×ÓŗĖĶāµÄM²ćÖŠÖ»ÓŠĮ½¶Ō³É¶Ōµē×Ó£¬YŌ×ÓµÄŗĖĶāL²ćµē×ÓŹżŹĒK²ćµÄĮ½±¶£¬ZŹĒµŲæĒÄŚŗ¬Įæ(ÖŹĮæ·ÖŹż)×īøßµÄŌŖĖŲ£¬QµÄŗĖµēŗÉŹżŹĒXÓėZµÄŗĖµēŗÉŹżÖ®ŗĶ£¬EŌŚŌŖĖŲÖÜĘŚ±ķµÄø÷ŌŖĖŲÖŠµēøŗŠŌ×ī“ó£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)XZ2µÄ·Ö×ÓŹ½ĪŖ__________£¬·Ö×ÓµÄĮ¢Ģå¹¹ŠĶĪŖ__________”£
(2)YZ2µÄµē×ÓŹ½ĪŖ__________£¬Ęä·Ö×ÓÖŠŗ¬ÓŠ__________Ģõ![]() ¼ü£¬__________Ģõ¦Š¼ü”£
¼ü£¬__________Ģõ¦Š¼ü”£
(3)QµÄŌŖĖŲ·ūŗÅŹĒ__________£¬ŹōÓŚ__________Ēų£¬ĖüµÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ__________”£¼Ūµē×ÓÅŲ¼Ķ¼ĪŖ__________£¬ŌŚŠĪ³É»ÆŗĻĪļŹ±Ėü×īøߵĻÆŗĻ¼ŪĪŖ__________”£
(4)EµÄµ„ÖŹÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”湤ŅµÉĻæÉŅŌÓĆ·ĻĢśŠ¼Öʱø»īŠŌFe3O4£¬Į÷³ĢČēĻĀĶ¼£ŗ
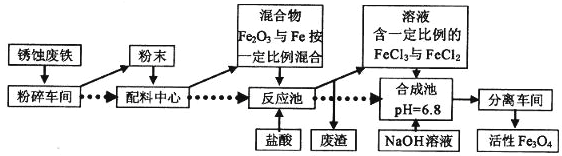
£Ø1£©ŌŚÖʱø¹ż³ĢÖŠ£¬ŅŖ½«æéד¹ĢĢåŌĮĻ·ŪĖ锢Ą³É·ŪÄ©£¬×÷ÓĆŹĒ________________________
£Ø2£©ŌŚŗĻ³É³ŲĄļÉś³ÉFe3O4µÄĄė×Ó·½³ĢŹ½ĪŖ__________________________
£Ø3£©¼ŁČēŌŚ·“Ó¦³ŲÖŠ¼øŗõƻӊĘųĢå²śÉś,øł¾ŻĮ÷³ĢæÉÖŖ£¬ÅäĮĻÖŠŠÄŗÜæÉÄÜŹ¹»ģŗĻĪļÖŠµÄFe2O3ÓėFeĪļÖŹµÄĮæÖ®±Č½Ó½ü________
£Ø4£©Ä³Ķ¬Ń§ĄūÓĆ·ĻĢśŠ¼£Øŗ¬FeŗĶFe2O3£©Ą“ÖĘČ”FeCl3”¤6H2O¾§Ģ壬Ķ¬Ź±²ā¶Ø»ģŗĻĪļÖŠĢśµÄÖŹĮæ·ÖŹż£¬×°ÖĆČēĶ¼£Ø¼Š³Ö×°ÖĆĀŌ£¬ĘųĆÜŠŌŅŃ¼ģŃ飩£ŗ

²Ł×÷²½ÖčČēĻĀ£ŗ
I£®“ņæŖµÆ»É¼ŠK1”¢¹Ų±ÕµÆ»É¼ŠK2£¬²¢“ņæŖ»īČūa£¬»ŗĀżµĪ¼ÓŃĪĖį”£
¢ņ£®µ±””Ź±£¬¹Ų±ÕµÆ»É¼ŠK1“ņæŖµÆ»É¼ŠK2£¬µ±AÖŠČÜŅŗĶźČ«½ųČėÉÕ±ŗó¹Ų±Õ»īČūa”£
¢ó£®½«ÉÕ±ÖŠČÜŅŗÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖŗóµĆµ½FeC13”¤6H2O¾§Ģ唣
Ēė»Ų“š£ŗ
¢Ł²Ł×÷¢ņÖŠ”°”””±µÄÄŚČŻŹĒ______________£¬ÉÕ±ÖŠµÄĻÖĻóŹĒ________________£¬ĻąÓ¦µÄ·½³ĢŹ½ŹĒ________________”¢________________”££ØŹĒĄė×Ó·“Ó¦µÄŠ“Ąė×Ó·½³ĢŹ½£©
¢ŚČō»ģŗĻĪļÖŹĮæĪŖm g£¬ŹµŃé½įŹųŗó²āµĆBÖŠĖłµĆµÄĘųĢåŹĒV mL£Ø±ź×¼×“æöŹ±£©£¬øĆĶ¬Ń§ÓÉ“Ė¼ĘĖć³ö“Ė·ĻĢśŠ¼ÖŠĢśµÄÖŹĮæ·ÖŹżŹĒ![]() £¬øĆŹżÖµ±ČŹµ¼ŹŹżÖµĘ«µĶ£¬ČōŹµŃé¹ż³Ģ²Ł×÷ĪŽĪó£¬Ę«µĶµÄŌŅņŹĒ______________________”£
£¬øĆŹżÖµ±ČŹµ¼ŹŹżÖµĘ«µĶ£¬ČōŹµŃé¹ż³Ģ²Ł×÷ĪŽĪó£¬Ę«µĶµÄŌŅņŹĒ______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ±ź×¼×“æöĻĀ£¬V L HClČܽāŌŚ1LĖ®ÖŠ£ØĖ®µÄĆÜ¶Č½üĖĘĪŖ1g/mL£©£¬ĖłµĆČÜŅŗµÄĆܶČĪŖ¦Ń g/mL£¬ÖŹĮæ·ÖŹżĪŖw£¬ĪļÖŹµÄĮæÅضČĪŖc mol/L£¬ŌņĻĀĮŠ¹ŲĻµÖŠ²»ÕżČ·µÄ£Ø £©
A.![]()
B.![]()
C.![]()
D.![]()
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĄūÓĆ”°»Æѧ¼ĘĮæŌŚŹµŃéÖŠµÄÓ¦ÓĆ”±µÄĻą¹ŲÖŖŹ¶ĢīæÕ”£
£Ø1£©ŗ¬ÓŠ6£®02”Į1023øöŃõŌ×ÓµÄH2SO4µÄĪļÖŹµÄĮæŹĒ___________”£
£Ø2£©Óė±ź×¼×“æöĻĀV LCO2Ėłŗ¬ŃõŌ×ÓŹżÄæĻąĶ¬µÄĖ®µÄÖŹĮæŹĒ___________g£ØÓĆ·ÖŹ½±ķŹ¾£©
£Ø3£©ÉčNAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£¬Čē¹ūa gŃõĘųÖŠŗ¬ÓŠµÄ·Ö×ÓŹżĪŖb£¬Ōņc gŃõĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żŌ¼ŹĒ________(ÓĆŗ¬NAµÄŹ½×Ó±ķŹ¾)”£
£Ø4£©½«4 g NaOHČܽāŌŚĖ®ÖŠÅä³É10 mLČÜŅŗ£¬ŌŁĻ”ŹĶ³É1 L£¬“ÓÖŠČ”³ö10 mL£¬Õā10 mLČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ_______”£
£Ø5£©Ģå»ż±ČĪŖ1”Ć2”Ć3µÄĀČ»ÆÄĘ”¢ĀČ»ÆĆ¾ŗĶĀČ»ÆĀĮČÜŅŗ£¬·Ö±š¼ÓČėµČĢå»ż”¢µČÅØ¶ČµÄĻõĖįŅųČÜŅŗ£¬¾łĒ”ŗĆĶźČ«·“Ӧɜ³ÉĀČ»ÆŅų³Įµķ£¬ŌņÕāČżÖÖČÜŅŗµÄĪļÖŹµÄĮæÅضČÖ®±ČĪŖ_________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com