
 ŅŃÖŖH2C2O4ĪŖ¶žŌŖÖŠĒæĖį£®Ä³»ÆѧŠĖȤŠ”×éĪŖ²ā¶Øŗ¬Na2SO4”¢NaHC2O4ŗĶH2C2O4•2H2OµÄŹŌŃłÖŠø÷ĪļÖŹµÄÖŹĮæ·ÖŹż£¬½ųŠŠĮĖČēĻĀŹµŃé£ŗ
ŅŃÖŖH2C2O4ĪŖ¶žŌŖÖŠĒæĖį£®Ä³»ÆѧŠĖȤŠ”×éĪŖ²ā¶Øŗ¬Na2SO4”¢NaHC2O4ŗĶH2C2O4•2H2OµÄŹŌŃłÖŠø÷ĪļÖŹµÄÖŹĮæ·ÖŹż£¬½ųŠŠĮĖČēĻĀŹµŃé£ŗ·ÖĪö £Ø1£©NaHC2O4ČÜŅŗÓėNaOHČÜŅŗ·“Ӧɜ³ÉNa2C2O4ŗĶĖ®£»
£Ø2£©ÅäÖĘŹŌŃłČÜŅŗŹ±ĖłŠčŅŖµÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢²£Į§°ō”¢ĮæĶ²”¢½ŗĶ·µĪ¹Ü”¢250mLČŻĮæĘ棻
£Ø3£©²½Öč¢ŪČōµĪ¶ØÖÕµćŹ±ČÜŅŗµÄpH=8.3£¬ŌņӦєŌń¼īŠŌÖøŹ¾¼Į£¬µĪ¶Ø¹Ü0æĢ¶ČŌŚÉĻĆę£¬ĆæŅ»øöŠ”æĢ¶ČĪŖ0.1mL£¬¾Ż“Ė“šĢā£»
£Ø4£©øł¾ŻµĪ¶Ø²Ł×÷ŅŖĒó£¬ŃŪ¾¦Ó¦¹Ū²ģ׶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ£»
£Ø5£©·“Ó¦ÖŠĢ¼“Ó+3¼ŪÉżĪŖ+4¼Ū£¬ĆĢ“Ó+7¼Ū½µĪŖ+2¼Ū£¬øł¾Ż»ÆŗĻÉż½µ·Ø¼°ŌŖĖŲŹŲŗćŗĶµēŗÉŹŲŗćÅäĘ½Ąė×Ó·½³ĢŹ½£»
£Ø6£©²½Öč¢ŪÖŠ£¬øßĆĢĖį¼ŲČÜŅŗÓŠĒæŃõ»ÆŠŌ£¬ÄÜŃõ»ÆČé½ŗ¹Ü£¬µĪ¶ØÖÕµćŹ±ČÜŅŗÓÉĪŽÉ«±ä³ÉĒ³ŗģÉ«£»
£Ø7£©Éčѳʷ֊NaHC2O4ĪŖxmol£¬H2C2O4•2H2OĪŖymol£¬øł¾ŻµŚŅ»·ŻČÜŅŗÖŠ¼Ó2”«3µĪÖøŹ¾¼Į£¬ÓĆ0.2500mol•L-1 NaOHČÜŅŗµĪ¶Ø£¬ĻūŗÄNaOHČÜŅŗ20.00mLæɵĆx+2y=0.2500”Į0.02mol=0.005mol£¬øł¾ŻµŚ¶ž·ŻČÜŅŗÓĆ0.1000mol•L-1µÄĖįŠŌøßĆĢĖį¼ŲČÜŅŗµĪ¶Ø£¬ĻūŗÄøßĆĢĖį¼ŲČÜŅŗ16.00mL£¬½įŗĻµē×ӵƏ§ŹŲŗć£¬æɵĆx+y=$\frac{5}{2}$”Į0.1000”Į0.016mol=0.004mol£¬¾Ż“Ė¼ĘĖć³öNaHC2O4ŗĶH2C2O4•2H2OµÄÖŹĮ棬ŌŁČ·¶ØŹŌŃłÖŠNa2SO4µÄÖŹĮæ·ÖŹż£®
½ā“š ½ā£ŗ£Ø1£©NaHC2O4ČÜŅŗÓėNaOHČÜŅŗ·“Ӧɜ³ÉNa2C2O4ŗĶĖ®£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖHC2O4-+OH-=H2O+C2O42-£¬
¹Ź“š°øĪŖ£ŗHC2O4-+OH-=H2O+C2O42-£»
£Ø2£©ÅäÖĘŹŌŃłČÜŅŗŹ±ĖłŠčŅŖµÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢²£Į§°ō”¢ĮæĶ²”¢½ŗĶ·µĪ¹Ü”¢250mLČŻĮæĘ棬
¹Ź“š°øĪŖ£ŗ½ŗĶ·µĪ¹Ü”¢250mLČŻĮæĘ棻
£Ø3£©²½Öč¢ŪČōµĪ¶ØÖÕµćŹ±ČÜŅŗµÄpH=8.3£¬ŌņӦєŌń¼īŠŌÖøŹ¾¼ĮĪŖ·ÓĢŖ£¬µĪ¶Ø¹Ü0æĢ¶ČŌŚÉĻĆę£¬ĆæŅ»øöŠ”æĢ¶ČĪŖ0.1mL£¬ĖłŅŌøł¾ŻĶ¼ÉĻµÄæĢ¶Č¹ÜÄŚŅŗĢåµÄĢå»żÓ¦ĪŖ“óÓŚ27.60mL£¬¹ŹŃ”d£¬
¹Ź“š°øĪŖ£ŗ·ÓĢŖ£»d£»
£Ø4£©øł¾ŻµĪ¶Ø²Ł×÷ŅŖĒó£¬ŃŪ¾¦Ó¦¹Ū²ģ׶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ£¬
¹Ź“š°øĪŖ£ŗ׶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ£»
£Ø5£©·“Ó¦ÖŠĢ¼“Ó+3¼ŪÉżĪŖ+4¼Ū£¬ĆĢ“Ó+7¼Ū½µĪŖ+2¼Ū£¬øł¾Ż»ÆŗĻÉż½µ·Ø¼°ŌŖĖŲŹŲŗćŗĶµēŗÉŹŲŗćæÉÖŖĄė×Ó·½³ĢŹ½ĪŖ5H2C2O4+2MnO4-+6H+=10CO2”ü+2Mn2++8H2O£¬
¹Ź“š°øĪŖ£ŗ5”¢2”¢6”¢10”¢2”¢8H2O£»
£Ø6£©²½Öč¢ŪÖŠ£¬øßĆĢĖį¼ŲČÜŅŗÓŠĒæŃõ»ÆŠŌ£¬ÄÜŃõ»ÆČé½ŗ¹Ü£¬ĖłŅŌøßĆĢĖį¼ŲČÜŅŗӦװŌŚĖįŹ½µĪ¶Ø¹ÜĄļ£¬ÅŠ¶ĻµĪ¶ØÖÕµćµÄ·½·ØŹĒČÜŅŗÓÉĪŽÉ«±äĪŖĒ³ŗģÉ«ĒŅŌŚ°ė·ÖÖÓÄŚ²»ĶŹÉ«£¬
¹Ź“š°øĪŖ£ŗĖįŹ½£»ČÜŅŗÓÉĪŽÉ«±äĪŖĒ³ŗģÉ«ĒŅŌŚ°ė·ÖÖÓÄŚ²»ĶŹÉ«£»
£Ø7£©Éčѳʷ֊NaHC2O4ĪŖxmol£¬H2C2O4•2H2OĪŖymol£¬øł¾ŻµŚŅ»·ŻČÜŅŗÖŠ¼Ó2”«3µĪÖøŹ¾¼Į£¬ÓĆ0.2500mol•L-1 NaOHČÜŅŗµĪ¶Ø£¬ĻūŗÄNaOHČÜŅŗ20.00mLæɵĆx+2y=0.2500”Į0.02mol=0.005mol£¬øł¾ŻµŚ¶ž·ŻČÜŅŗÓĆ0.1000mol•L-1µÄĖįŠŌøßĆĢĖį¼ŲČÜŅŗµĪ¶Ø£¬ĻūŗÄøßĆĢĖį¼ŲČÜŅŗ16.00mL£¬½įŗĻµē×ӵƏ§ŹŲŗć£¬æɵĆx+y=$\frac{5}{2}$”Į0.1000”Į0.016mol=0.004mol£¬ĖłŅŌ$\left\{\begin{array}{l}{x+2y=0.005\\;}\\{x+y=0.004}\end{array}\right.$£¬½āµĆx=0.003£¬y=0.001£¬ĖłŅŌ10.0gŹŌŃłÖŠŗ¬ÓŠNaHC2O4µÄÖŹĮæĪŖ$\frac{250}{25}$”Į112”Į0.003g=3.36g£¬H2C2O4•2H2OµÄÖŹĮæĪŖ$\frac{250}{25}$”Į126”Į0.001g=1.26g£¬ŌņŹŌŃłÖŠNa2SO4µÄÖŹĮæ·ÖŹżĪŖ$\frac{10-1.26-3.36}{10}$”Į100%=53.8%£¬
¹Ź“š°øĪŖ£ŗ53.8%£®
µćĘĄ ±¾Ģāæ¼²éŃõ»Æ»¹ŌµĪ¶ØŌĄķÓėÓ¦ÓĆ”¢Ģ½¾æÓ°ĻģĖŁĀŹµÄŅņĖŲ£¬ÄѶČÖŠµČ£¬Ąķ½āŹµŃéŌĄķŹĒ½āĢāµÄ¹Ų¼ü£¬ŹĒ¶ŌÖŖŹ¶µÄ×ŪŗĻŌĖÓĆ£¬ŠčŅŖѧɜ¾ß±øŌśŹµµÄ»ł“”ÖŖŹ¶ÓėŌĖÓĆÖŖŹ¶·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāµÄÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| Ī¢Į£ | ÖŹ×ÓŹż | ÖŠ×ÓŹż | ÖŹĮæŹż | ×īĶā²ćµē×ÓŹż | ZAX |
| Al | 27 | ||||
| S2- | 1634S2- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆ¼īŹ½µĪ¶Ø¹Ü×¼Č·ĮæČ”20.00 mLµÄøßĆĢĖį¼ŲČÜŅŗ | |
| B£® | ÓĆNaOHµĪ¶ØŃĪĖįŹ±£¬ČōµĪ¶Ø½įŹųŹ±ø©ŹÓæĢ¶Č£¬»įµ¼ÖĀ²ā¶Ø½į¹ūĘ«øß | |
| C£® | ÓĆNaOHµĪ¶ØŃĪĖįŹ±£¬Ö»ÄÜÓĆ·ÓĢŖ×÷ÖøŹ¾¼Į | |
| D£® | ÓĆKMnO4µĪ¶ØŃĒĮņĖįÄĘČÜŅŗµÄŹµŃéÖŠ²»ŠčŅŖĮķĶā¼ÓČėÖøŹ¾¼Į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
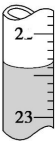
 Ä³ŃŠ¾æŠŌѧĻ°Š”×éĒėÄć²ĪÓė”°ŃŠ¾æĢśÓėĖ®·“Ó¦ĖłµĆ¹ĢĢåĪļÖŹµÄ³É·Ö”¢ŠŌÖŹ¼°ŌŁĄūÓĆ”±ŹµŃéĢ½¾æ£¬²¢¹²Ķ¬½ā“šĻĀĮŠĪŹĢā£ŗ
Ä³ŃŠ¾æŠŌѧĻ°Š”×éĒėÄć²ĪÓė”°ŃŠ¾æĢśÓėĖ®·“Ó¦ĖłµĆ¹ĢĢåĪļÖŹµÄ³É·Ö”¢ŠŌÖŹ¼°ŌŁĄūÓĆ”±ŹµŃéĢ½¾æ£¬²¢¹²Ķ¬½ā“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
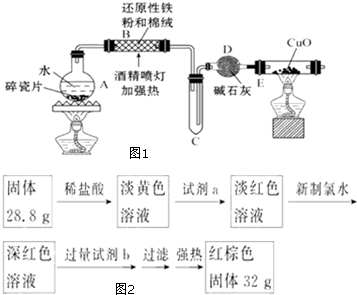
 Ņ»¶ØĢõ¼žĻĀ£¬ĶعżĻĀĮŠ·“Ó¦æÉŹµĻÖČ¼ĆŗŃĢĘųÖŠĮņµÄ»ŲŹÕ£ŗ
Ņ»¶ØĢõ¼žĻĀ£¬ĶعżĻĀĮŠ·“Ó¦æÉŹµĻÖČ¼ĆŗŃĢĘųÖŠĮņµÄ»ŲŹÕ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com