
(分)下图表示有关物质(均是中学化学常见的物质)之间的转化关系(其
中部分参加反应的水和生成的水未列出),其中B、C为金属单质,E为非金属单质,其余均为化合物,Y、Z的组成元素相同,D为淡黄色的固体。回答系列问题:
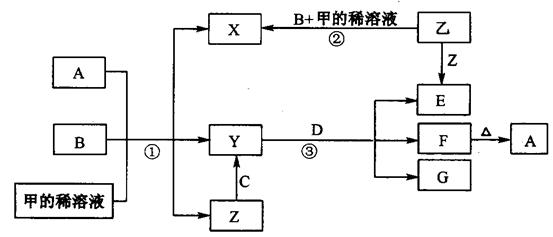
(1)B与甲稀溶液不反应,但与甲的浓溶液在加热条件下能反应。则甲为 ,C为 (填写化学式)。
A与B物质的量应满足的关系为 。
(2)写出反应③的化学方程式 。
(3)反应②观察到溶液由无色变成蓝色,并且有无色气泡产生。请写出反应②发生的化学反应方程式 ,并分析产生气体的原因 。
 七星图书口算速算天天练系列答案
七星图书口算速算天天练系列答案 初中学业考试导与练系列答案
初中学业考试导与练系列答案科目:高中化学 来源: 题型:
下图表示有关物质(均由短周期元素形成)之间的转化关系,其中A为常见的金属单
![]() 质,B为非金属单质(一般是黑色粉末),C是常见的无色无味液体,D是淡黄色的固体化
质,B为非金属单质(一般是黑色粉末),C是常见的无色无味液体,D是淡黄色的固体化
![]() 合物。(反应条件图中已省略。)
合物。(反应条件图中已省略。)
![]()
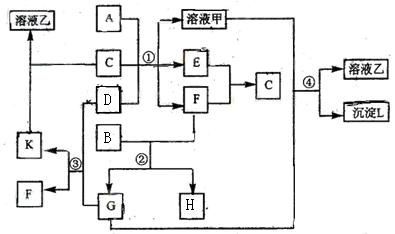
![]() (1)A、B、C、D代表的物质分别为 、 、 、 (填化学式);
(1)A、B、C、D代表的物质分别为 、 、 、 (填化学式);
![]() (2)反应①中的C、D均过量,该反应的化学方程式是 ;
(2)反应①中的C、D均过量,该反应的化学方程式是 ;
![]() (3)反应②中,若B与F物质的量之比为4∶3,G、H分别是 、 (填化学式);
(3)反应②中,若B与F物质的量之比为4∶3,G、H分别是 、 (填化学式);
![]() (4)反应③产物中K的化学式为 ;
(4)反应③产物中K的化学式为 ;
![]() (5)反应④的离子方程式为 。
(5)反应④的离子方程式为 。
查看答案和解析>>
科目:高中化学 来源: 题型:
(6分) 下图是由短周期元素组成的一些单质及其化合物之间的转化关系图。各方框表示有关的一种反应物或生成物(某些物质已经略去),其中A、B、D在常温下均为无色无刺激性气味的气体,C是使湿润的红色石蕊试纸变蓝的气体,M是最常见的无色液体。
⑴物质G的化学式: 。
⑵物质B的电子式: 。
⑶写出C→E的化学方程式: ;
⑷G→F的离子方程式: 。
查看答案和解析>>
科目:高中化学 来源:2012年江苏省泰州中学高二学业水平测试模拟(3) 化学试卷 题型:填空题
(6分) 下图是由短周期元素组成的一些单质及其化合物之间的转化关系图。各方框表示有关的一种反应物或生成物(某些物质已经略去),其中A、B、D在常温下均为无色无刺激性气味的气体,C是使湿润的红色石蕊试纸变蓝的气体,M是最常见的无色液体。
⑴物质G的化学式: 。
⑵物质B的电子式: 。
⑶写出C→E的化学方程式: ;
⑷G→F的离子方程式: 。
查看答案和解析>>
科目:高中化学 来源:2012年江苏省高二学业水平测试模拟(3)化学试卷 题型:填空题
(6分) 下图是由短周期元素组成的一些单质及其化合物之间的转化关系图。各方框表示有关的一种反应物或生成物(某些物质已经略去),其中A、B、D在常温下均为无色无刺激性气味的气体,C是使湿润的红色石蕊试纸变蓝的气体,M是最常见的无色液体。

⑴物质G的化学式: 。
⑵物质B的电子式: 。
⑶写出C→E的化学方程式: ;
⑷G→F的离子方程式: 。
查看答案和解析>>
科目:高中化学 来源: 题型:
下图中的每一个方格表示有关的一种反应物或生成物。已知X为一种盐,其分
解产物中A、B、C的物质的量之比为1:1:1,A为无色无味气体。试完成下列问题:
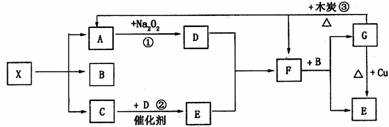
(1)写出下列物质的化学式
X ,F
(2)写出下列反应的化学方程式
反应① ,
反应② ,
反应③ 。
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com