
��10�֣���1��ʵ����������������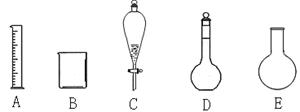
�������������ѡ���ʵ���������Ҫ����ա�
��a���������ֻ������ܵ�Һ���������__ ������ĸ����
��b������250ml0.1 mol��L-1��������Ҫ�õ���������_ ������ĸ����
��c������������һ�����ʵ���Ũ�ȵ�������_ _ �����������ƣ���
��d��������ʱ������ˮҺ��û���̶��ߣ���ҺŨ�Ƚ�_ (ƫ�ߡ�ƫ�͡���Ӱ��)��
(2)�ڵ��۵⻯����Һ��ͨ�����������������ῴ����Һ����ɫ��������Ϊ_______________________________________________(���û�ѧ����ʽ���Լ�Ҫ���ֽ��н���)
��10�֣�ÿ��2�֣�(a) C (b) ABD (c) ����ƿ��d��ƫ��
��2���⻯�ر��������������˵��ʵ⣬��Ӧ�����ӷ���ʽ��Cl2��2I��=2Cl����I2��
���������������1���������ֻ������ܵ�Һ��������Ƿ�Һ©������ѡC������250ml0.1 mol��L-1��������Ҫ�õ����������������ձ�������ƿ�����Դ�ѡABD������������һ�����ʵ���Ũ�ȵ�����������ƿ������c��n/V��֪��������ʱ������ˮҺ��û���̶��ߣ�����Һ�����ƫ����ҺŨ�Ƚ�ƫ�͡�
��2��������������ɫ����˵����Ӧ�е⻯�ر��������������˵��ʵ⣬��Ӧ�����ӷ���ʽ��Cl2��2I��=2Cl����I2��
���㣺���鳣��������ʹ�á����ʵ���Ũ�����Ƶ��������Լ����ӷ���ʽ����д
����������cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�������������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
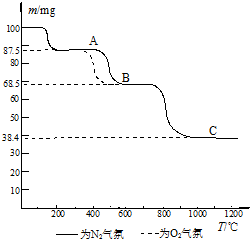
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
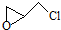 ������л���˴Ź���������
������л���˴Ź���������
| I | - 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
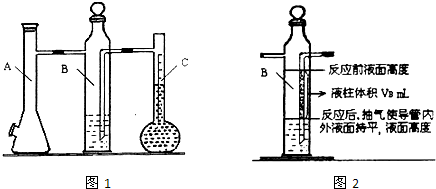
| ʵ����� | m��Mg��/g | �������/mL | Һ����ƿ��Һ�����/mL | ����������/mL | �������/mL | ����1mol�����/L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | X | |
| 2 | 0.115 | 10.0 | 121.0 | 8.0 |
| ʵ����� | m��Mg�� g |
�������mL | Һ����ƿ��Һ�����mL | ����������mL | Bƿ��һ��Һ�����mL | ˮ������ٷֺ��� | ����1mol�����L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | VB | a% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com