
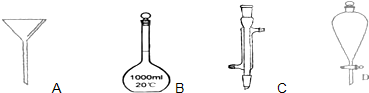
��11�֣������Ǽ���ʵ���г��õ�������
 A
A  B
B  C
C  D
D
��1��д����������������������ƣ�
B___________��C___________��D___________
ʵ����Ҫ����500 mL 0.2 mol/L NaOH��Һ����ش��������⣺
��2�����ƹ���������Ҫʹ�õĻ�ѧ������__________________����ѡ�����ĸ����
A���ձ����� B��500 mL����ƿ���� C��©������ D����ͷ�ι� ������E��������
��3����������ƽ��ȡ�������ƣ�������Ϊ_______________g��
��4��������Ҫ�����������ȷ˳����_________������ţ���
�ٳ�ȡһ���������������ƣ������ձ��У�����������ˮ�ܽ⣻
�ڡ����ݡ�
�۴���ȴ�����º���Һת�Ƶ�500 mL ����ƿ�У�
�ܸǺ�ƿ�����������µߵ���ҡ�ȣ�
��������������ˮϴ���ձ��ڱںͲ�����2~3�Σ�ϴ��Һת�Ƶ�����ƿ�С�
��5��д������ڵľ��������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ����������ʦ���и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
I�������Ǽ���ʵ���г��õ�������

д����������������������ƣ�
A____________;B____________;C_____________;D______________��
��ʵ����Ҫ����480 mL, O��2 mol/L NaOH��Һ����ش��������⣺
��1�����ƹ����в���Ҫʹ�õĻ�ѧ������___________����ѡ�����ĸ����
A���ձ� B��500 mL����ƿ C��©�� D����ͷ�ι� E��������
��2����������ƽ��ȡ�������ƣ�������Ϊ_______________g��
��3��ȡ����������ĸ�NaOH��Һʱ�������������в�����ȡ����Ķ��ٶ��仯����________________________��
A����Һ��NaOH�����ʵ��� B����Һ��Ũ��
C����Һ�е���Ŀ D����Һ���ܶ�
��4��������������Һ�Ĺ����У����������NaOH��Һ���ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�������Ӱ�족��

��δ����ȴ���Ƚ���Һע������ƿ�У�__________________��
������ƿ������ϴ�Ӻ������������ˮ��_______________��
�۶���ʱijͬѧ�۲�Һ��������ͼ��ʾ���������õ���Һ��Ũ_______________��
��ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ���______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ��������У��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
�����Ǽ���ʵ���г��õ�������
A. B.
B. C.
C. D.
D.
��1��д����������������������ƣ�B___________��C___________��D___________
ʵ����Ҫ����500 mL 0.2 mol/L NaOH��Һ����ش��������⣺
��2����������ƽ��ȡ�������ƣ�������Ϊ_______________g��
��3��������Ҫ�����������ȷ˳����_______________������ţ���
�ٳ�ȡһ���������������ƣ������ձ��У�����������ˮ�ܽ⣻
�ڡ����ݡ�
�۴���ȴ�����º���Һת�Ƶ�500 mL ����ƿ�У�
�ܸǺ�ƿ�����������µߵ���ҡ�ȣ�
��������������ˮϴ���ձ��ڱںͲ�����2��3�Σ�ϴ��Һת�Ƶ�����ƿ�С�
��4�������ƹ����У���������������ȷ�ģ����в�������������Ũ��ƫ�ߵ���__________��
��û��ϴ���ձ��Ͳ����� ��ת����Һʱ������������������ƿ����
������ƿ�����������������ˮ �ܶ���ʱ���ӿ̶���
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
��5��д������ڡ����ݡ��ľ��������
��6������ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ijѧ����
��С�����Ӻ�������ȡ�⣬������µ����̣�
����֪���̢��з�Ӧ�����ӷ���ʽΪ��2I�� + Cl2 = 2Cl�� + I2��

��.ָ����ȡ��Ĺ������йص�ʵ����������ƣ��� �� �� ��
��.��ȡ��Ĺ����У���ѡ����л��ܼ��� (�����) ��
A���ƾ� B������ C �����Ȼ�̼
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com