
ĶĘ½ųĘ÷ÖŠŹ¢ÓŠĒ滹Ō¼ĮėĀ£ØN2H4£©ŗĶĒæŃõ»Æ¼ĮŅŗĢ¬Ė«ŃõĖ®”£µ±ĖüĆĒ»ģŗĻ·“Ó¦Ź±£¬¼“²śÉś“óĮæµŖĘųŗĶĖ®ÕōĘų£¬²¢·Å³ö“óĮæČČ”£ŅŃÖŖ0.4molŅŗĢ¬ėĀÓė×ćĮæŅŗĢ¬Ė«ŃõĖ®·“Ó¦£¬Éś³ÉµŖĘųŗĶĖ®ÕōĘų£¬·Å³ö256.652KJµÄČČĮ攣
£Ø1£©Š“³ö¹żŃõ»ÆĒāµÄµē×ÓŹ½ ”£
£Ø2£©øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
1molėĀĶźČ«·“Ó¦×ŖŅʵē×ÓŹż ”£
£Ø3£©“Ė·“Ó¦ÓĆÓŚ»š¼żĶĘ½ų£¬³żŹĶ·Å“óĮæČČŗĶæģĖŁ²śÉś“óĮæĘųĢåĶā£¬»¹ÓŠŅ»øöŗÜ“óµÄÓŵćŹĒ ”£
£Ø4£©ÓÖŅŃÖŖH2O(l)==H2O(g)£»”÷H = +44kJ?mol-1£¬ÓÉ16gŅŗĢ¬ėĀÓėŅŗĢ¬Ė«ŃõĖ®·“Ӧɜ³ÉŅŗĢ¬Ė®Ź±·Å³öµÄČČĮæŹĒ kJ”£
£Ø1£©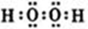 £Ø2£©N2H4£Øl£©+2H2O2£Øl£©ØTN2£Øg£©+4H2O£Øg£© ”÷H=-641.6KJ/mol£»4NA
£Ø2£©N2H4£Øl£©+2H2O2£Øl£©ØTN2£Øg£©+4H2O£Øg£© ”÷H=-641.6KJ/mol£»4NA
£Ø3£©Éś³ÉĪļĪŽĪŪČ¾ £Ø4£©408.8
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©¹żŃõ»ÆĒā·Ö×ÓÖŠĒāŌ×ÓÓėŃõŌ×ÓÖ®¼äŠĪ³É¼«ŠŌ¼ü£¬ŃõŌ×ÓÓėŃõŌ×ÓÖ®¼äŠĪ³É·Ē¼«ŠŌ¼ü£¬µē×ÓŹ½ĪŖ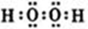 ”£
ӣ
£Ø2£©ŅŃÖŖ0.4molŅŗĢ¬ėĀŗĶ×ćĮæĖ«ŃõĖ®·“Ӧɜ³ÉµŖĘųŗĶĖ®ÕōĘųŹ±·Å³ö256.652KJµÄČČĮ棻ėĀŗĶĖ«ŃõĖ®·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗN2H4£Øl£©+2H2O2£Øl£©ØTN2£Øg£©+4H2O£Øg£© ”÷H=-641.6KJ/mol”£ėĀ·Ö×ÓÖŠµŖŌŖĖŲµÄ»ÆŗĻ¼ŪŹĒ£2¼Ū£¬·“Ó¦ŗó±äĪŖ0¼Ū£¬Ź§Č„2øöµē×Ó£¬Ōņ1molėĀĶźČ«·“Ó¦×ŖŅʵē×ÓŹżĪŖ4NA”£
£Ø3£©»¹Ō¼ĮėĀ£ØN2H4£©ŗĶĒæŃõ»Æ¼ĮH2O2£¬µ±ĖüĆĒ»ģŗĻŹ±£¬¼“²śÉś“óĮæµÄµŖĘųŗĶĖ®ÕōĘų£¬³żŹĶ·Å“óĮæČČĮæŗĶæģĖŁ²śÉś“óĮæĘųĢåĶā£¬»¹ÓŠŗÜĶ»³öµÄÓŵćŹĒ£¬²śĪļĪŖµŖĘųŗĶĖ®£¬ĪŽĪŪČ¾£»¹Ź“š°øĪŖ£ŗ²śĪļĪŖµŖĘųŗĶĖ®£¬ĪŽĪŪČ¾£»
£Ø4£©¢ŁN2H4£Øl£©+2H2O2£Øl£©ØTN2£Øg£©+4H2O£Øg£©£»”÷H=-641.6KJ/mol£»¢ŚH2O£Øl£©=H2O£Øg£©£»”÷H=+44KJ/mol£¬ŅĄ¾ŻøĒĖ¹¶ØĀÉ¢Ł-¢Ś”Į4µĆµ½£ŗN2H4£Øl£©+2H2O2£Øl£©ØTN2£Øg£©+4H2O£ØL£©£»”÷H=-817.6KJ/mol£»»Æѧ·½³ĢŹ½ÖŠ32gČ«²æ·“Ó¦·ÅČČ817.6KJ£¬16gŅŗĢ¬ėĀÓė×ćĮæĖ«ŃõĖ®·“Ӧɜ³ÉµŖĘųŗĶŅŗĢ¬Ė®Ź±£¬·Å³öµÄČČĮæŹĒ408.8KJ”£
æ¼µć£ŗæ¼²éČČ»Æѧ·½³ĢŹ½µÄŹéŠ“·½·ØŗĶ×¢ŅāĪŹĢā£¬øĒĖ¹¶ØĀɵÄÓ¦ÓĆ£¬ČČ»Æѧ·½³ĢŹ½µÄ¼ĘĖć



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ČōNAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
| A£®1 mol-OHÖŠŗ¬ÓŠµÄÖŹ×ÓŹżĪŖ9NA |
| B£®lmol2£¬3”Ŗ¶”¶ž“¼·Ö×ÓÖŠŗ¬CŅ»CŹżÄæĪŖ4NA |
| C£®1 L 1 mol.L-1FeCl3ČÜŅŗÖŠŗ¬ÓŠ Fe3 +ŹżĪŖNA |
| D£®71 gĀČĘų²ĪÓė·“Ó¦£¬µē×Ó×ŖŅĘŹżÄæŅ»¶ØĪŖ2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠø÷ĻīÖŠ±ķ“ļÕżČ·µÄŹĒ£Ø £©
| A£®CO2µÄ½į¹¹Ź½£ŗO”ŖC”ŖO |
B£®ŅŅĻ©·Ö×ÓĒņ¹÷Ä£ŠĶ£ŗ |
C£®NaClµÄµē×ÓŹ½£ŗ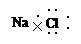 |
D£®F”ŖµÄ½į¹¹Ź¾ŅāĶ¼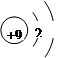 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©Ä³Ķ¬Ń§°“ĻĀĮŠ²½ÖčÅäÖĘ500 mL 0.2 mol”¤L£1 KClČÜŅŗ£¬Ēė»Ų“šÓŠ¹ŲĪŹĢā”£
| ŹµŃé²½Öč | ÓŠ¹ŲĪŹĢā |
| ¢Ł¼ĘĖćĖłŠčKClµÄÖŹĮæ | ŠčŅŖKClµÄÖŹĮæĪŖ________g(±£ĮōŠ”ŹżµćŗóŅ»Ī») |
| ¢Ś³ĘĮæKCl¹ĢĢå | ³ĘĮæŠčŅŖÓƵ½µÄÖ÷ŅŖŅĒĘ÷ŹĒ________________ |
| ¢Ū½«KCl¼ÓČė100 mLÉÕ±ÖŠ£¬²¢¼ÓČėŹŹĮæĖ® | ĪŖĮĖ¼ÓæģČܽāĖŁĀŹ£¬æÉŅŌ²ÉČ”ÄÄŠ©“ėŹ©£æ ________________ |
| ¢Ü½«ÉÕ±ÖŠČÜŅŗ×ŖŅĘÖĮ500 mLČŻĮæĘæÖŠ | ĪŖĮĖ·ĄÖ¹ČÜŅŗ½¦³ö£¬Ó¦²ÉČ”Ź²Ć““ėŹ©£æ __________________ |
| ¢ŻĻņČŻĮæĘæÖŠ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻß | ŌŚ½ųŠŠ“Ė²Ł×÷Ź±µ±¼ÓĖ®ÖĮĄėæĢ¶ČĻß1 cm”«2 cm“¦Ó¦ČēŗĪ²Ł×÷£æ____________________ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø6·Ö£©ÓĆ9mol/LµÄÅØĮņĖįĻ”ŹĶ³É 0£®9mol/LµÄĻ”ĮņĖį 100mL £¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÅäÖĘ²Ł×÷æÉ·Ö½ā³ÉČēĻĀ¼ø²½£¬ŅŌĻĀÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ_____________________
A ĻņČŻĮæĘæ֊עČėÉŁĮæÕōĮóĖ®£¬¼ģ²éŹĒ·ńĀ©Ė®
B ÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±¼°²£Į§°ō£¬½«ČÜŅŗ×¢ČėČŻĮæĘ棬²¢ÖŲø“²Ł×÷Į½“Ī
C ÓĆŅŃĄäČ“µÄĻ”ĮņĖį×¢ČėŅŃ¼ģ²é²»Ā©Ė®µÄČŻĮæĘæÖŠ
D øł¾Ż¼ĘĖć£¬ÓĆĮæĶ²ĮæČ”Ņ»¶ØĢå»żµÄÅØĮņĖį
E£®½«ÅØĮņĖįŃŲÉÕ±±ŚĀżĀż×¢ČėŹ¢ÓŠÕōĮóĖ®µÄŠ”ÉÕ±ÖŠ£¬²¢²»¶ĻÓĆ²£Į§°ō½Į°č
F£®øĒÉĻČŻĮæĘæČū×Ó£¬Õńµ“£¬Ņ”ŌČ
G£®ÓĆ½ŗĶ·µĪ¹ÜµĪ¼ÓÕōĮóĖ®£¬Ź¹ČÜŅŗ°¼ĆęĒ”ŗĆÓėæĢ¶ČĻąĒŠ
H£®¼ĢŠųĶłČŻĮæĘæÖŠŠ”ŠÄµŲ¼ÓÕōĮóĖ®£¬Ź¹ŅŗĆę½Ó½üæĢ¶ČĻß1~2 cm
£Ø2£©Čē¹ūŹµŃéŹŅÓĆ98£„µÄÅØĮņĖį(ĆܶČĪŖ1£®8g”¤cm-3 ) ÅäÖĘ3£® 6 mol”¤L-1µÄĻ”ĮņĖį250mL”£¼ĘĖćĖłŠčÅØĮņĖįµÄĢå»żĪŖ_____________mL”£
£Ø3£©ÓÉÓŚ“ķĪó²Ł×÷, Ź¹µĆµ½µÄÅØ¶ČŹż¾Ż±ČÕżČ·µÄĘ«“óµÄŹĒ___________£ØĢīŠ“ŠņŗÅ£©”£
A Ź¹ÓĆČŻĮæĘæÅäÖĘČÜŅŗŹ±, ø©ŹÓŅŗĆę¶ØČŻŗóĖłµĆČÜŅŗµÄÅضČ
B ƻӊÓĆÕōĮóĖ®Ļ“ÉÕ±2-3“Ī£¬²¢½«Ļ“ŅŗŅĘČėČŻĮæĘæÖŠ
C ČŻĮæĘæÓĆÕōĮóĖ®Ļ“¾»£¬Ć»ÓŠŗęøÉ
D ¶ØČŻŹ±£¬µĪ¼ÓÕōĮóĖ®£¬ĻČŹ¹ŅŗĆęĀŌøßÓŚæĢ¶ČĻߣ¬ŌŁĪü³öÉŁĮæĖ®Ź¹ŅŗĆę°¼ĆęÓėæĢ¶ČĻßĻąĒŠ
E£®°ŃÅäŗƵÄČÜŅŗµ¹ČėÓĆÕōĮóĖ®Ļ“¾»¶ųĪ“øɵďŌ¼ĮĘæÖŠ±øÓĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø5·Ö£©
£Ø1£©ŌŚĻĀĮŠ±ä»ÆÖŠ£ŗ¢ŁµāµÄÉż»Ŗ ¢ŚÉÕ¼īČŪ»Æ ¢ŪMgCl2ČÜÓŚĖ® ¢ÜHClČÜÓŚĖ®,
Ī“·¢Éś»Æѧ¼üĘĘ»µµÄŹĒ £¬½ö·¢ÉśĄė×Ó¼üĘĘ»µµÄŹĒ ”££ØĢīŠ“ŠņŗÅ£©
£Ø2£©ĻĀĮŠĪåÖÖĪļÖŹÖŠ¢ŁNe ¢ŚNa2O¢ŪNH3¢ÜKOH£¬Ö»“ęŌŚ¹²¼Ū¼ü
µÄŹĒ £¬¼Č“ęŌŚ¹²¼Ū¼üÓÖ“ęŌŚĄė×Ó¼üµÄŹĒ ”££ØĢīŠ“ŠņŗÅ£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijµŖ·ŹNH4HCO3ÖŠ»ģÓŠÉŁĮæ(NH4)2CO3£¬ĻÖ²ÉÓĆĻĀĮŠ·½°ø²ā¶ØøƵŖ·ŹÖŠ(NH4)2CO3µÄÖŹĮæ·ÖŹż£ŗ³ĘČ”5.7 gѳʷÓė2.0 mol”¤L£1 NaOHČÜŅŗ»ģŗĻ£¬ĶźČ«Čܽāŗ󣬵ĶĪĀ¼ÓČČŹ¹Ęä³ä·Ö·“Ó¦(øĆĪĀ¶ČĻĀļ§ŃĪ²»·Ö½ā)£¬²¢Ź¹Éś³ÉµÄ°±ĘųČ«²æ±»ĮņĖįĪüŹÕ£¬²āµĆ°±ĘųµÄÖŹĮæÓėĖłÓĆNaOHČÜŅŗĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ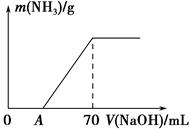
(1)AµćĒ°ŃłĘ·ÓėNaOH·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
(2)ĪŖŹ¹Éś³ÉµÄ°±Ęų±»ĮņĖįĪüŹÕŹ±²»·¢Éśµ¹Īü£¬æÉŅŌŃ”ÓĆĻĀĮŠ×°ÖĆÖŠµÄ (Ģī×ÖÄøŠņŗÅ)”£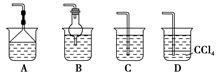
(3)ѳʷ֊(NH4)2CO3µÄÖŹĮæ·ÖŹżŹĒ %(±£ĮōŅ»Ī»Š”Źż)”£
(4)µ±V(NaOH)£½50 mLŹ±£¬Éś³ÉNH3µÄÖŹĮæĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅÅäÖĘ480 mL 0£®600 mol”¤L£1µÄNaClČÜŅŗ£¬ÓŠČēĻĀ²Ł×÷²½Öč£ŗ
¢Ł°Ń³ĘĮæŗƵÄNaCl¾§Ģå·ÅČėŠ”ÉÕ±ÖŠ£¬¼ÓŹŹĮæÕōĮóĖ®Čܽā
¢Ś°Ń¢ŁĖłµĆČÜŅŗŠ”ŠÄ×ŖČėČŻĮæĘæÖŠ
¢Ū¼ĢŠųĻņČŻĮæĘæÖŠ¼ÓÕōĮóĖ®ÖĮŅŗĆę¾ąæĢ¶ČĻß1 cm”«2 cm“¦£¬øÄÓĆ½ŗĶ·µĪ¹ÜŠ”ŠÄµĪ¼ÓÕōĮóĖ®ÖĮČÜŅŗ°¼ŅŗĆęÓėæĢ¶ČĻßĻąĒŠ
¢ÜÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±ŗĶ²£Į§°ō2”«3“Ī£¬Ćæ“ĪĻ“µÓµÄŅŗĢ嶼Š”ŠÄ×ŖČėČŻĮæĘ棬²¢ĒįĒįŅ”ŌČ
¢Ż½«ČŻĮæĘæĘæČūČū½ō£¬³ä·ÖŅ”ŌČ
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)²Ł×÷²½ÖčµÄÕżČ·Ė³ŠņĪŖ(ĢīŠņŗÅ) ”£
(2)ŹµŃéŹŅÓŠČēĻĀ¹ęøńµÄČŻĮæĘæ£ŗ¢Ł100 mL£¬¢Ś250 mL£¬¢Ū500 mL£¬¢Ü1000 mL£¬±¾ŹµŃéŃ”ÓĆ(ĢīŠņŗÅ) ”£
(3)±¾ŹµŃéÓƵ½µÄ»ł±¾ŹµŃéŅĒĘ÷³żČŻĮæĘ攢²£Į§°ōĶā£¬»¹ÓŠ ”£
(4)ŠčŅŖŹ¹ÓĆ²£Į§°ōµÄ²Ł×÷ÓŠ (Ģī²½ÖčŠņŗÅ)£¬Ęä×÷ÓĆĪŖ ”£
(5)Īó²ī·ÖĪö£ŗ(Ģī”°Ę«øß”±”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±)
¢Ł³ĘĮæNaClŹ±£¬ĪļĀėµ¹ÖĆ(1 gŅŌĻĀÓĆÓĪĀė) ”£
¢ŚÄ³Ķ¬Ń§¹Ū²ģŅŗĆęµÄĒéæöČēÓŅĶ¼ĖłŹ¾£¬¶ŌĖłÅäČÜŅŗÅØ¶Č½«ÓŠŗĪÓ°Ļģ ”£
¢Ūƻӊ½ųŠŠ²Ł×÷²½Öč¢Ü ”£
¢Ü¼ÓÕōĮóĖ®Ź±²»É÷³¬¹żĮĖæĢ¶ČĻߣ¬Į¢¼“ÓĆ½ŗĶ·µĪ¹Ü½«¶ąÓąµÄĖ®Īü³ö ”£
¢ŻČŻĮæĘæÖŠŌĄ“ÓŠÉŁĮæĖ® ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°±ŗĶĮŖ°±(N2H4)ŹĒµŖµÄĮ½ÖÖ³£¼ū»ÆŗĻĪļ£¬ŌŚæĘѧ¼¼ŹõŗĶÉś²śÖŠÓŠÖŲŅŖµÄÓ¦ÓĆ”£øł¾ŻĢāŅāĶź³ÉĻĀĮŠ¼ĘĖć£ŗ
(1)ĮŖ°±ÓĆŃĒĻõĖįŃõ»ÆÉś³ÉµŖµÄĮķŅ»ÖÖĒā»ÆĪļ£¬øĆĒā»ÆĪļµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ43.0£¬ĘäÖŠµŖŌ×ÓµÄÖŹĮæ·ÖŹżĪŖ0.977£¬¼ĘĖćČ·¶ØøĆĒā»ÆĪļµÄ·Ö×ÓŹ½ĪŖ________”£øĆĒā»ÆĪļŹÜײ»÷ŌņĶźČ«·Ö½āĪŖµŖĘųŗĶĒāĘų”£4.30 gøĆĒā»ÆĪļŹÜײ»÷ŗó²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ________L”£
(2)ĮŖ°±ŗĶĖÄŃõ»Æ¶žµŖæÉÓĆ×÷»š¼żĶĘ½ų¼Į£¬ĮŖ°±ŹĒČ¼ĮĻ£¬ĖÄŃõ»Æ¶žµŖ×öŃõ»Æ¼Į£¬·“Ó¦²śĪļŹĒµŖĘųŗĶĖ®”£ÓÉĮŖ°±ŗĶĖÄŃõ»Æ¶žµŖ×é³ÉµÄ»š¼żĶĘ½ų¼ĮĶźČ«·“Ӧɜ³É72.0 kgĖ®£¬ŌņĶĘ½ų¼ĮÖŠĮŖ°±µÄÖŹĮæ________”£
(3)°±µÄĖ®ČÜŅŗæÉÓĆÓŚĪüŹÕNOÓėNO2»ģŗĻĘųĢ壬·“Ó¦·½³ĢŹ½ĪŖ6NO£« 4NH3=5N2£«6H2O ””6NO2£« 8NH3=7N2£«12H2O”£NOÓėNO2»ģŗĻĘųĢå180 mol±»8.90”Į103g°±Ė®(ÖŹĮæ·ÖŹż0.300)ĶźČ«ĪüŹÕ£¬²śÉś156 molµŖĘų”£ĪüŹÕŗó°±Ė®ĆܶČĪŖ0.980 g/cm3”£Ōņ¢ŁøĆ»ģŗĻĘųĢåÖŠNOÓėNO2µÄĢå»ż±ČĪŖ________£¬¢ŚĪüŹÕŗó°±Ė®µÄĪļÖŹµÄĮæÅضČ________(“š°ø±£Įō1Ī»Š”Źż)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com