
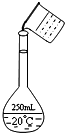
 ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ������ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩
ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ������ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ����ǣ����㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ����ѡ��ʹ�õ�������
��2������������Һ���ѡ������ƿ�����ҺʱӦ�ò�����������
��3������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��4��������Һ�����Ʋ����ϸ������Ĺ��켰ʹ�÷��������жϣ�
��5��ʵ��������Ҫ����2mol/L��NaOH��Һ950mL������û��950mL������ƿ��ʵ������ʱ��Ҫѡ��1000mL����ƿ�����Ƶ���ҺΪ1000mL 2mol/L��NaOH��Һ������ʵ�����Ƶ�����������Һ�������Ũ�ȼ�����������Ƶ��������ݴ˽��
��� �⣺��1������һ�����ʵ���Ũ�ȵ���Һ�IJ����ǣ����㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȿ�֪��Ҫ�������У�������ƽ��ҩ�ס��ձ�����������500mL����ƿ�ͽ�ͷ�ιܣ�û��ʹ�õ���������Ʒ�Тڣ���ȱ�ٵ������ǽ�ͷ�ιܣ�
�ʴ�Ϊ���ڣ���ͷ�ιܣ�
��2���ù���NaOH����0.5mol/L��NaOH��Һ500mL��Ӧѡ��500mL����ƿ����ҺʱӦ�ò�����������
�ʴ�Ϊ������ƿ���ѡ�������Һû�ò�����������
��3��A��û�н�ϴ��Һת�Ƶ�����ƿ���������ʲ�����ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Aѡ��
B��ת�ƹ�������������Һ�������������ʲ�����ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Bѡ��
C������ƿϴ����δ�������Һ��������ʵ����ʵ������������Ӱ�죬��ҺŨ�Ȳ��䣬��C��ѡ��
D������ʱ���ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���D��ѡ��
��ѡ��AB��
��4��A��ʹ������ƿǰ������Ƿ�©ˮ������������Һ��Ũ����������ȷ��
B������ƿ������ˮϴ�������ü�Һ��ϴ�������������ʵ���ƫ����ҺŨ��ƫ�ߣ��ʴ���
C������ƿΪ��������������ʢ�Ź���Һ�壬�������ƹ����ܽ�ų��������ȣ�Ӧ��ȴ������Һ����C����
D�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ�ȣ���D��ȷ��
��ѡ��BC��
��5��ʵ��������Ҫ����2mol/L��NaOH��Һ950mL������û��950mL������ƿ��ʵ������ʱ��Ҫѡ��1000mL����ƿ�����Ƶ���ҺΪ1000mL 2mol/L��NaOH��Һ����Ҫ�������Ƶ�����m=cVM=1L��2mol/L��40g/mol=80.0g��
��ѡ��A��
���� ���⿼����������ƿ����һ�����ʵ���Ũ����Һ��ʵ�������ע�������ȷ����ԭ����ʵ����������ǽ���ؼ���ע������ƿ�����ص㼰ʹ��ע�����⣬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Na2CO3��Һ�У�c��OH-��-c��H+��=c��HCO3-��+c��H2CO3�� | |
| B�� | 25��ʱpH=10��NaOH��Һ��pH=10�İ�ˮ�У�c��Na+��=c��NH4+�� | |
| C�� | 25��ʱpH=9��Ũ�Ⱦ�Ϊ0.1mol/L��NH3•H2O��NH4Cl�����Һ��c��NH4+��+c��H+����c��NH3•H2O��+c��OH-�� | |
| D�� | 0.1mol/L��NH4��2Fe��SO4��2��Һ�У�c��SO42-��=c��NH4+����c��Fe2+����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��I-��AlO2-��MnO4- | B�� | Na+��S2-��NO3-��SO42- | ||
| C�� | Al3+��NH4+��NO3-��F- | D�� | K+��Cl-��ClO-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe2O3�����������Һ�У�Fe2O3+6H+�T2Fe3++3H2O | |
| B�� | AlƬ����NaOH��Һ�У��������壺2Al+2OH-+2H2O�T2AlO2-+3H2�� | |
| C�� | Ca��HCO3��2��Һ������NaOH��Һ��Ӧ��HCO3-+Ca2++OH-�TCaCO3��+H2O | |
| D�� | ��Cu2O�����м���������ϡ���Cu2O+2H+�TCu+Cu2++H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | A | B | C | D |
| ʵ����� | ��MgCl2��AlCl3��Һ�У���1mol������μ���NaOH��Һ | ��HCl��MgCl2��AlCl3��NH4Cl��Һ�У���1mol������μ���NaOH��Һ | ��NaOH��NaAlO2��Һ�У���1mol������μ���HCl��Һ | ��NaOH��Na2CO3�����Һ�У���1mol���μ�ϡ���� |
| ͼ�� | 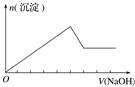 |  | 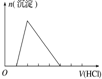 | 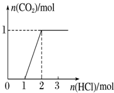 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����������� | ���� |
| A | �ò�˿պȡij����Һ�ھƾ������������գ��������ɫ������ɫ�ܲ����� | ������Һ��һ�����м�Ԫ�أ� ���ܺ���Ԫ�� |
| B | ��ij��ɫ��Һ��ͨ�������CO2���壬�а�ɫ�������� | ����Һ��һ������SiO32- |
| C | ��Ʒ����Һ��ͨ��ij�������Һ��ɫ | ������һ����SO2 |
| D | ��NaOH��Һ�еμ�MgCl2��Һ��������ɫ�����������μ�FeCl3��Һ�������ɫ���� | Fe��OH��3���ܽ�ȴ���Mg��OH��2���ܽ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ư�۵���Ҫ�ɷ�Ϊ������� | |
| B�� | ʵ���ҿ���Ũ������ﰱ�� | |
| C�� | ʵ���ҿ���NaOH��Һ����NO2��HCl���� | |
| D�� | ���Ƕ�Ӧ�ĺ����ᶼ��ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| Fe3+ | Cu2+ | Cr3+ | |
| ��ʼ����pH | 2.1 | 4.7 | 4.3 |
| ��ȫ����pH | 3.2 | 6.7 | a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com