
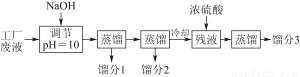
Β―ι “άϊ”ΟΝρΥα≥ß…’‘ϋΘ®÷ς“Σ≥…Ζ÷ΈΣΧζΒΡ―θΜ·ΈοΦΑ…ΌΝΩFeSΓΔSiO2Β»Θ©÷Τ±ΗΨέΧζΘ®Φν ΫΝρΥαΧζΒΡΨέΚœΈοΘ©ΚΆ¬ΧΖ·Θ®FeSO4ΓΛ7H2OΘ©Θ§Ιΐ≥Χ»γœ¬ΘΚ

Θ®1Θ©ΫΪΙΐ≥ΧΔΎ÷–≤ζ…ζΒΡΤχΧεΆ®»κœ¬Ν–»ή“Κ÷–Θ§»ή“ΚΜαΆ …ΪΒΡ «________ΓΘ
AΘ°ΤΖΚλ»ή“Κ BΘ°Ήœ…Ϊ ·»ο»ή“Κ
CΘ°Υα–‘KMnO4»ή“Κ DΘ°δεΥ°
Θ®2Θ©Ιΐ≥ΧΔΌ÷–Θ§FeSΚΆO2ΓΔH2SO4Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_____________________________ΓΘ
Θ®3Θ©Ιΐ≥ΧΔέ÷–Θ§–ηΦ”»κΒΡΈο÷ «________ΓΘ
Θ®4Θ©Ιΐ≥ΧΔή÷–Θ§’τΖΔΫαΨß–η“Σ Ι”ΟΨΤΨΪΒΤΓΔ»ΐΫ≈ΦήΓΔΡύ»ΐΫ«Θ§ΜΙ–η“ΣΒΡ“«Τς”–________________ΓΘ
Θ®5Θ©Ιΐ≥ΧΔίΒςΫΎpHΩ…―Γ”Οœ¬Ν– ‘ΦΝ÷–ΒΡ________Θ®Χν―Γœν–ρΚ≈Θ©ΓΘ
AΘ°œΓΝρΥα BΘ°CaCO3 CΘ°NaOH»ή“Κ
Θ®6Θ©Ιΐ≥ΧΔό÷–Θ§ΫΪ»ή“ΚZΦ”»»ΒΫ70ΓΪ80 ΓφΘ§ΡΩΒΡ «______________________ΓΘ
Θ®7Θ© Β―ι “ΈΣ≤βΝΩΥυΒΟΒΫΒΡΨέΧζ―υΤΖ÷–Χζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΘ§Ϋχ––œ¬Ν– Β―ιΓΘ
ΔΌ”ΟΖ÷ΈωΧλΤΫ≥Τ»Γ2Θ°700 g―υΤΖΘΜ
ΔΎΫΪ―υΤΖ»ή”ΎΉψΝΩΒΡ―ΈΥαΚσΘ§Φ”»κΙΐΝΩΒΡ¬»Μ·±Β»ή“ΚΘΜ
Θ®1Θ©ACD
Θ®2Θ©4FeSΘΪ3O2ΘΪ6H2SO4=2Fe2Θ®SO4Θ©3ΘΪ6H2OΘΪ4S
Θ®3Θ©FeΘ®ΜρΧζΘ©ΓΓΘ®4Θ©’τΖΔΟσΓΔ≤ΘΝßΑτΓΓΘ®5Θ©B
Θ®6Θ©¥ΌΫχFe3ΘΪΒΡΥ°Ϋβ
Θ®7Θ©31Θ°1%
ΓΨΫβΈωΓΩΘ®1Θ©Ιΐ≥ΧΔΎ÷–≤ζ…ζΒΡΤχΧε «SO2Θ§ΨΏ”–Τ·ΑΉ–‘Θ®Ρή ΙΤΖΚλ»ή“ΚΆ …ΪΘ©ΘΜSO2Ε‘ΥαΦν÷Η ΨΦΝΟΜ”–Τ·ΑΉΉς”ΟΓΘ
Θ®2Θ©FeSΜ·ΚœΦέ…ΐΗΏΈΣ1ΘΪ2ΘΫ3, O2 Μ·ΚœΦέΫΒΒΆ4Θ§ΝΫ’ΏΗω ΐ±» «4ΓΟ3Θ§‘ΌΫαΚœ‘≠Ή” ΊΚψ≈δΤΫΓΘ
Θ®3Θ©Ιΐ≥ΧΔέ÷–Φ”»κΧζΫΪFe3ΘΪΜΙ‘≠ΈΣFe2ΘΪΘ§”÷≤Μ“ΐ»κ‘”÷ ΓΘ
Θ®4Θ©’τΖΔ»ή“Κ ±ΫΪ“ΚΧεΖ≈‘Ύ’τΖΔΟσ÷–Θ§≤Δ≤ΜΕœ”Ο≤ΘΝßΑτΫΝΑηΓΘ
Θ®5Θ©÷–ΚΆΙΐΝΩΝρΥαΘ§―Γ‘ώCaCO3ΙΧΧεΘ§»τ―Γ‘ώNaOH »ή“ΚΘ§‘ρ“Ή ΙFe3ΘΪ≥ΝΒμΓΘ
Θ®6Θ©…ΐΗΏΈ¬Ε»Θ§¥ΌΫχFe3ΘΪΒΡΥ°ΫβΓΘ
Θ®7Θ©”…“―÷ΣΩ…ΒΟmΘ®BaSO4Θ©ΘΫ3Θ°495 gΘ§‘ρnΘ®SO42-Θ©ΘΫ0Θ°015 molΘ§ΗυΨίΉι≥…÷ΣnΘ®FeΘ©ΘΫnΘ®SO42-Θ©ΘΫ0Θ°015 molΘ§mΘ®FeΘ©ΘΫ0Θ°84 gΘ§Χζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΈΣ0Θ°84 gΓ¬2Θ°700 gΓΝ100%ΘΫ31Θ°1%ΓΘ


 » Α°”Δ”οΆ§≤ΫΝΖœΑ≤αœΒΝ–¥πΑΗ
» Α°”Δ”οΆ§≤ΫΝΖœΑ≤αœΒΝ–¥πΑΗ ―ßœΑ ΒΦυ‘ΑΒΊœΒΝ–¥πΑΗ
―ßœΑ ΒΦυ‘ΑΒΊœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑœό ±Φ·―Β Ή®Χβ16Έο÷ ΫαΙΙ”κ–‘÷ ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
–μΕύΫπ τΦΑΥϋΟ«ΒΡΜ·ΚœΈο‘ΎΩΤ―ß―–ΨΩΚΆΙΛ“Β…ζ≤ζ÷–ΨΏ”––μΕύ”ΟΆΨΓΘ
ΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
Θ®1Θ©ΜυΧ§NiΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ__________________________________________Θ§
ΒΎΕΰ÷ήΤΎΜυΧ§‘≠Ή”Έ¥≥…Ε‘ΒγΉ” ΐ”κNiœύΆ§«“ΒγΗΚ–‘Ήν–ΓΒΡ‘ΣΥΊ «________ΓΘ
Θ®2Θ©≈δΚœΈοNiΘ®COΘ©nΒΡ÷––Ρ‘≠Ή”ΦέΒγΉ” ΐ”κ≈δΧεΧαΙ©ΒγΉ”Ήή ΐ÷°ΚΆΈΣ18Θ§‘ρnΘΫ________Θ§CO”κN2ΫαΙΙœύΥΤΘ§COΖ÷Ή”ΡΎΠ“Φϋ”κΠ–ΦϋΗω ΐ÷°±»ΈΣ________ΓΘ
Θ®3Θ©NiOΓΔFeOΒΡΨßΧεΫαΙΙάύ–ΆΨυ”ꬻ̷ΡΤΒΡœύΆ§ΓΘ
ΔΌNi2ΘΪΚΆFe2ΘΪΒΡάκΉ”ΑκΨΕΖ÷±πΈΣ69 pmΚΆ78 pmΘ§‘ρ»έΒψNiO________FeOΘ®ΧνΓΑ<Γ±ΜρΓΑ>Γ±Θ©ΘΜ
ΔΎNiOΨßΧε÷–NiΒΡ≈δΈΜ ΐΈΣ________ΓΘ
Θ®4Θ©Ϋπ τCuΒΞΕά”κΑ±Υ°ΜρΒΞΕά”κΙΐ―θΜ·«βΕΦ≤ΜΡήΖ¥”ΠΘ§ΒΪΩ…”κΑ±Υ°ΚΆΙΐ―θΜ·«βΒΡΜλΚœ»ή“ΚΖ¥”ΠΘ§Τδ‘≠“ρ «________________________________________________________________________________________________________________________________________________Θ§
Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ________________________________________________________________________ΓΘ
Θ®5Θ©“Μ÷÷Ά≠ΫπΚœΫπΨßΧεΨΏ”–Οφ–ΡΝΔΖΫΉνΟήΕ―ΜΐΒΡΫαΙΙΓΘ‘ΎΨßΑϊ÷–Θ§Au‘≠Ή”ΈΜ”ΎΕΞΒψΘ§Cu‘≠Ή”ΈΜ”ΎΟφ–ΡΘ§‘ρΗΟΚœΫπ÷–Au‘≠Ή””κCu‘≠Ή”Ηω ΐ÷°±»ΈΣ________Θ§»τΗΟΨßΑϊΒΡ±Ώ≥ΛΈΣa pmΘ§‘ρΚœΫπΒΡΟήΕ»ΈΣ________gΓΛcmΘ≠3Θ®÷Μ“Σ«σΝ–Υψ ΫΘ§≤Μ±ΊΦΤΥψ≥ω ΐ÷ΒΘ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐΈΣNAΘ©ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑœό ±Φ·―Β Ή®Χβ12”–ΜζΈοΒΡΫαΙΙ”κ–‘÷ ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
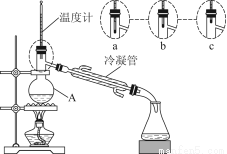
Ρ≥―ßœΑ–ΓΉι”ϊΡΘΡβ¥”Ρ≥ΙΛ≥ßΖœ“Κ÷–ΜΊ ’±ϊΆΣΓΔ““¥ΦΚΆ““ΥαΒΡ Β―ιΓΘ÷ΤΕ®ΝΥ»γœ¬ ‘―ιΝς≥ΧΓΘ
“―÷ΣΗΟΖœ“Κ÷–÷ς“ΣΚ§”–““¥ΦΘ§Τδ÷–ΜΙ»ή”–±ϊΆΣΓΔ““ΥαΚΆ““Υα““θΞΓΘ«“Ης÷÷≥…Ζ÷ΒΡΖ–Βψ»γœ¬±μΘΚ
Έο÷ | ±ϊΆΣ | ““Υα““θΞ | ““¥Φ | ““Υα |
Ζ–ΒψΘ®ΓφΘ© | 56.2 | 77.06 | 78 | 117.9 |
Θ®1Θ©ΝσΖ÷3ΒΡ≥…Ζ÷ΈΣ____________ΓΘ
Θ®2Θ©…œ ωΝς≥Χ÷–ΒςΫΎpHΘΫ10ΒΡΡΩΒΡ «________________________________________________________________________________________________________________________________________________ΓΘ
Θ®3Θ©ΗΟ–ΓΉιΆ§―ßΒΡ’τΝσΉΑ÷Ο»γΆΦΥυ ΨΓΘ‘ρA÷–Έ¬Ε»ΦΤΒΡΈΜ÷Ο’ΐ»ΖΒΡ «________Θ®ΧνΓΑaΓ±ΓΑbΓ±ΜρΓΑcΓ±Θ©ΓΘ
Θ®4Θ©ΙζΦ“±ξΉΦΙφΕ®Θ§”≈÷ ΗΏΕ»≈®œψ–ΆΑΉΨΤΉήΥαΝΩΘ®“‘““ΥαΦΤΘ©”Π≤Μ…Ό”Ύ0.30 g/LΘ§ΉήθΞΝΩΘ®“‘““Υα““θΞΦΤΘ©”Π≤Μ…Ό”Ύ2.0 g/LΓΘ
ΔΌΈΣ≤βΕ®Ρ≥ΑΉΨΤ―υΤΖΒΡΉήΥαΝΩΘ§»Γ20.00 mL―υΤΖ”ΎΉΕ–ΈΤΩ÷–Θ§Φ”»κΖ”ΧΣ÷Η ΨΦΝ2ΒΈΘ§”Ο0.010 mol/LΒΡNaOH±ξΉΦ»ή“ΚΒΈΕ®÷Ν÷’ΒψΓΘ≈–Εœ÷’ΒψΒΡ“άΨί «________________________________________________________________________________________________________________________________________________ΓΘ
»τΗΟΑΉΨΤ―υΤΖΈΣ”≈÷ ΦΕΘ§‘ρœϊΚΡNaOH»ή“ΚΧεΜΐ”Π≤Μ–Γ”Ύ________mLΓΘ
ΔΎΑΉΨΤ÷–ΒΡΉήθΞΝΩΩ…”ΟΖΒΒΈΖ®≤βΕ®ΓΘΆυ…œΧβΒΈΕ®ΚσΒΡ»ή“ΚΘ®«ΓΚΟ÷Ν÷’ΒψΘ©÷–‘ΌΦ”»κ20.00mL0.100mol/L NaOH±ξΉΦ»ή“ΚΘ§”ΟΆΦΉΑ÷ΟΥ°‘ΓΦ”»»Ακ–Γ ±ΓΘά以Κσ”Ο0.100mol/LΒΡΝρΥα±ξΉΦ»ή“ΚΒΈΕ®÷Ν÷’ΒψΓΘΦ”»»Ακ–Γ ±ΒΡΡΩΒΡ «______________________Θ§άδΡΐΙήΒΡΉς”Ο «______________ΓΘ“―÷ΣΉν÷’œϊΚΡΝρΥα±ξΉΦ»ή“Κ7.70 mLΘ§ΗΟΑΉΨΤ―υΤΖ÷–ΉήθΞΝΩΈΣ________g/LΘ®±ΘΝτ–Γ ΐΒψΚσ»ΐΈΜ ΐΉ÷Θ©ΓΘ
Θ®5Θ©œ¬Ν–≤ΌΉςΜα ΙΉήθΞΝΩ≤βΕ®ΫαΙϊΤΪΗΏΒΡ «________Θ®―ΓΧν±ύΚ≈Θ©
aΘ°Φ”»» ±Έ¥ Ι”ΟΥ°‘ΓΚΆάδΡΐΙή
bΘ°ΒΈΕ®«ΑΒΈΕ®ΙήΡΎΈόΤχ≈ίΘ§ΒΈΕ®Κσ≤ζ…ζΤχ≈ί
cΘ°ΒΈΕ®ΙήΈ¥”ΟΝρΥα±ξΉΦ»ή“Κ»σœ¥
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑœό ±Φ·―Β Ή®Χβ11Ϋπ τ‘ΣΥΊΒΞ÷ ΦΑΜ·ΚœΈοΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Κœ≥…Α±ΙΛ“Β…ζ≤ζ÷–Υυ”ΟΒΡΠΝ?Fe¥ΏΜ·ΦΝΒΡ÷ς“Σ≥…Ζ÷ «FeOΓΔFe2O3ΓΘ
Θ®1Θ©Ρ≥FeOΓΔFe2O3ΜλΚœΈο÷–Θ§ΧζΓΔ―θΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ4ΓΟ5Θ§Τδ÷–Fe2ΘΪ”κFe3ΘΪΈο÷ ΒΡΝΩ÷°±»ΈΣ________ΓΘ
Θ®2Θ©Β±¥ΏΜ·ΦΝ÷–Fe2ΘΪ”κFe3ΘΪΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ1ΓΟ2 ±Θ§Τδ¥ΏΜ·Μν–‘ΉνΗΏΘ§¥Υ ±ΧζΒΡ―θΜ·ΈοΜλΚœΈο÷–ΧζΒΡ÷ ΝΩΖ÷ ΐΈΣ________Θ®”Ο–Γ ΐ±μ ΨΘ§±ΘΝτ2ΈΜ–Γ ΐΘ©ΓΘ
Θ®3Θ©“‘Fe2O3ΈΣ‘≠Νœ÷Τ±Η…œ ω¥ΏΜ·ΦΝΘ§Ω…œρΤδ÷–Φ”»κ ΝΩΧΦΖέΘ§ΖΔ…ζ»γœ¬Ζ¥”ΠΘΚ2Fe2O3ΘΪC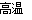 4FeOΘΪCO2ΓϋΓΘ
4FeOΘΪCO2ΓϋΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑœό ±Φ·―Β Ή®Χβ11Ϋπ τ‘ΣΥΊΒΞ÷ ΦΑΜ·ΚœΈοΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©
AΘ°Ϋπ τΡΤ‘ΎΩ’Τχ÷–»Φ…’ΒΡΜπ―φ≥ ΜΤ…ΪΘ§ «“ρΈΣ…ζ≥…ΒΡNa2O2ΈΣΒ≠ΜΤ…ΪΙΧΧε
BΘ°ΧζΥΩ‘ΎCl2÷–»Φ…’”–ΚλΉΊ…ΪΒΡ―ΧΘ§ «“ρΈΣ…ζ≥…ΒΡFeCl3ΈΣΚλΉΊ…ΪΙΧΧε
CΘ°ΙΐΝΩΒΡΧζΦ”»κœΓHNO3Θ§≥δΖ÷Ζ¥”ΠΚσΘ§ΒΈ»κKSCN»ή“ΚΘ§»ή“Κ≥ Κλ…ΪΘ§ΥΒΟςœΓHNO3ΫΪFe―θΜ·ΈΣFe3ΘΪ
DΘ°»Γ…ΌΝΩ»ή“ΚXΘ§œρΤδ÷–Φ”»κ ΝΩ–¬÷ΤΒΡ¬»Υ°Θ§‘ΌΦ”ΦΗΒΈKSCN»ή“ΚΘ§»ή“Κ±δΚλΘ§ΥΒΟςX»ή“Κ÷–“ΜΕ®Κ§”–Fe2ΘΪ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑœό ±Φ·―Β Ή®Χβ10Ζ«Ϋπ τ‘ΣΥΊΒΞ÷ ΦΑΜ·ΚœΈοΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©
AΘ°Cl2ΓΔSO2ΨυΡή ΙΤΖΚλ»ή“ΚΆ …ΪΘ§ΥΒΟςΕΰ’ΏΨυ”–―θΜ·–‘
BΘ°Ρή Ι Σ»σΒΡΒμΖέKI ‘÷Ϋ±δ≥…άΕ…ΪΒΡΈο÷ “ΜΕ® «Cl2
CΘ°SiO2”κΥαΓΔΦνΨυ≤ΜΖ¥”Π
DΘ°Ζ÷±π≥δ¬ζHClΓΔNH3ΒΡ…’ΤΩΒΙ÷Ο”ΎΥ°÷–Κσ“ΚΟφΨυ―ΗΥΌ…œ…ΐΘ§ΥΒΟςΕΰ’ΏΨυ“Ή»ή”ΎΥ°
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®ΧβΥΡ―θΜ·ΜΙ‘≠Ζ¥”ΠΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Θ®1Θ©ΫΪΖœΖΑ¥ΏΜ·ΦΝ(÷ς“Σ≥…Ζ÷V2O5)”κœΓΝρΥαΓΔ―«ΝρΥαΦΊ»ή“ΚΜλΚœΘ§≥δΖ÷Ζ¥”ΠΘ§ΥυΒΟ»ή“Κœ‘Υα–‘Θ§Κ§VO2ΘΪΓΔKΘΪΓΔSO42-Β»ΓΘ–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_________________________________ΓΘ
Θ®2Θ©œρ…œ ωΥυΒΟ»ή“Κ÷–Φ”»κKClO3»ή“ΚΘ§≥δΖ÷Ζ¥”ΠΚσΘ§»ή“Κ÷––¬‘ωΦ”ΝΥVO2+ΓΔClΘ≠ΓΘ–¥≥ω≤Δ≈δΤΫΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘ§≤Δ±ξ≥ωΒγΉ”ΉΣ“ΤΒΡ ΐΡΩΚΆΖΫœρ______________________ΓΘ
Θ®3Θ©‘Ύ20.00 mLΒΡ0.1 molΓΛLΘ≠1 VO2+»ή“Κ÷–Θ§Φ”»κ0.195 g –ΩΖέΘ§«ΓΚΟΆξ≥…Ζ¥”ΠΘ§‘ρΜΙ‘≠≤ζΈοΩ…Ρή «______________________________________________________________ΓΘ
aΘ°V bΘ°V2ΘΪ cΘ°VO2+ dΘ°VO2ΘΪ
Θ®4Θ©“―÷ΣV2O5ΡήΚΆ―ΈΥαΖ¥”Π…ζ≥…¬»ΤχΚΆVO2ΘΪΓΘ«κ‘Ό–¥“ΜΗωάκΉ”Ζ¥”ΠΖΫ≥Χ ΫΘ§ΥΒΟςΜΙ‘≠–‘ΘΚSO32-ΘΨClΘ≠ΘΨVO2ΘΪ__________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®Χβ °ΒγΫβ÷ »ή“ΚΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
≥ΘΈ¬œ¬Θ§œρ20.00 mL 0.100 molΓΛLΘ≠1 CH3COONa»ή“Κ÷–÷πΒΈΦ”»κ0.100 0 molΓΛLΘ≠1―ΈΥαΘ§»ή“ΚΒΡpH”κΥυΦ”―ΈΥαΧεΜΐΒΡΙΊœΒ»γœ¬ΆΦΥυ Ψ(≤ΜΩΦ¬«Μ”ΖΔ)ΓΘœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «(ΓΓΓΓ)

AΘ°ΒψΔΌΥυ Ψ»ή“Κ÷–ΘΚc(CH3COOH)ΘΫc(ClΘ≠)ΘΨc(OHΘ≠)ΘΫc(HΘΪ)
BΘ°ΒψΔΎΥυ Ψ»ή“Κ÷–ΘΚc(NaΘΪ)ΘΨc(ClΘ≠)ΘΨc(CH3COOΘ≠)ΘΨc(CH3COOH)
CΘ°ΒψΔέΥυ Ψ»ή“Κ÷–ΘΚc(CH3COOH)ΘΨc(NaΘΪ)ΘΨc(HΘΪ)ΘΨc(CH3COOΘ≠)
DΘ°’ϊΗωΙΐ≥Χ÷–Ω…Ρή≥ωœ÷ΘΚc(HΘΪ)ΘΪc(NaΘΪ)ΘΫc(CH3COOH)ΘΪc(CH3COOΘ≠)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®Χβ °Εΰ≥ΘΦϊΖ«Ϋπ τ‘ΣΥΊΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–”–ΙΊΈο÷ ΒΡ–‘÷ ”κ”Π”Ο≤ΜœύΕ‘”ΠΒΡ «(ΓΓΓΓ)
AΘ°Na2O2ΡήΖ÷±π”κH2OΓΔCO2Ζ¥”ΠΘ§Ω…”ΟΉςΙ©―θΦΝ
BΘ°ΙηΫΚΕύΩΉΓΔΈϋΥ°ΡήΝΠ«ΩΘ§≥Θ”ΟΉς¥ϋΉΑ ≥ΤΖΒΡΗ…‘οΦΝ
CΘ°K2FeO4ΨΏ”–«ΩΜΙ‘≠–‘«“±Μ―θΜ·…ζ≥…Fe3ΘΪΘ§Ω…”Ο”ΎΥ°ΒΡœϊΕΨΚΆΨΜΜ·
DΘ°“ΚΑ±ΤϊΜ· ±ΡήΈϋ ’¥σΝΩΒΡ»»Θ§ Ι÷ήΈßΈ¬Ε»Φ±ΨγΫΒΒΆΘ§“ρ¥ΥΩ…”ΟΉς÷ΤάδΦΝ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com