
| ||
| V”Į10-3L |
| 22.4L/mol |
| V |
| 22400 |
| m |
| Mr |
| V |
| 22400 |
| 11200a |
| V |
| 11200a |
| V |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
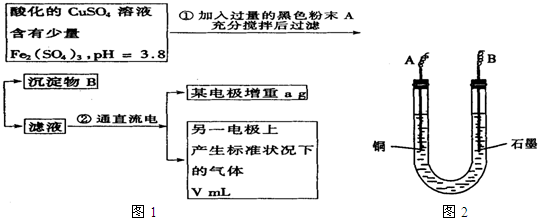
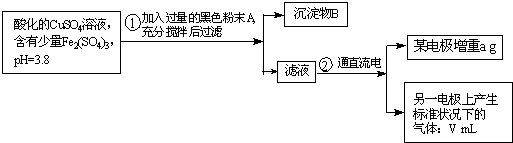
 ŅŃÖŖŌŚpHĪŖ4”«5µÄČÜŅŗÖŠ£¬Cu2+¼øŗõ²»Ė®½ā£¬¶ųFe3+¼øŗõĶźČ«Ė®½ā£®Ä³Ń§ÉśÄāÓƵē½āCuSO4ČÜŅŗµÄ·½·Ø²ā¶ØĶµÄĻą¶ŌŌ×ÓÖŹĮ森øĆĶ¬Ń§ĻņpH=3.8Ėį»ÆµÄ”¢ŗ¬ÓŠFe2£ØSO4£©3ŌÓÖŹµÄCuSO4ČÜŅŗÖŠ¼ÓČė¹żĮæµÄŗŚÉ«·ŪÄ©X£¬³ä·Ö½Į°čŗó½«ĀĖŅŗÓĆĻĀĶ¼ĖłŹ¾×°ÖƵē½ā£¬Ęä֊ijµē¼«ŌöÖŲa g£¬ĮķŅ»µē¼«ÉĻ²śÉś±ź×¼×“æöĻĀµÄĘųĢåVmL£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ŅŃÖŖŌŚpHĪŖ4”«5µÄČÜŅŗÖŠ£¬Cu2+¼øŗõ²»Ė®½ā£¬¶ųFe3+¼øŗõĶźČ«Ė®½ā£®Ä³Ń§ÉśÄāÓƵē½āCuSO4ČÜŅŗµÄ·½·Ø²ā¶ØĶµÄĻą¶ŌŌ×ÓÖŹĮ森øĆĶ¬Ń§ĻņpH=3.8Ėį»ÆµÄ”¢ŗ¬ÓŠFe2£ØSO4£©3ŌÓÖŹµÄCuSO4ČÜŅŗÖŠ¼ÓČė¹żĮæµÄŗŚÉ«·ŪÄ©X£¬³ä·Ö½Į°čŗó½«ĀĖŅŗÓĆĻĀĶ¼ĖłŹ¾×°ÖƵē½ā£¬Ęä֊ijµē¼«ŌöÖŲa g£¬ĮķŅ»µē¼«ÉĻ²śÉś±ź×¼×“æöĻĀµÄĘųĢåVmL£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŅŃÖŖŌŚpHĪŖ4”«5µÄČÜŅŗÖŠ£¬Cu2+¼øŗõ²»Ė®½ā£¬¶ųFe3+¼øŗõĶźČ«Ė®½ā£®Ä³Ķ¬Ń§ÄāÓƵē½āĮņĖįĶČÜŅŗµÄ·½·Ø²ā¶ØĶµÄĻą¶ŌŌ×ÓÖŹĮ森øĆĶ¬Ń§ĻņpH=3.8µÄŗ¬ÓŠĮņĖįĢśŌÓÖŹµÄĮņĖįĶČÜŅŗÖŠ¼ÓČė¹żĮæµÄŗŚÉ«·ŪÄ©X£¬³ä·Ö½Į°čŗó½«ĀĖŅŗÓĆČēĶ¼ĖłŹ¾×°ÖƵē½ā£¬Ęä֊ijµē¼«ŌöÖŲa g£¬ĮķŅ»µē¼«ÉĻ²śÉś±ź×¼×“æöĻĀµÄĘųĢåV mL£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ŅŃÖŖŌŚpHĪŖ4”«5µÄČÜŅŗÖŠ£¬Cu2+¼øŗõ²»Ė®½ā£¬¶ųFe3+¼øŗõĶźČ«Ė®½ā£®Ä³Ķ¬Ń§ÄāÓƵē½āĮņĖįĶČÜŅŗµÄ·½·Ø²ā¶ØĶµÄĻą¶ŌŌ×ÓÖŹĮ森øĆĶ¬Ń§ĻņpH=3.8µÄŗ¬ÓŠĮņĖįĢśŌÓÖŹµÄĮņĖįĶČÜŅŗÖŠ¼ÓČė¹żĮæµÄŗŚÉ«·ŪÄ©X£¬³ä·Ö½Į°čŗó½«ĀĖŅŗÓĆČēĶ¼ĖłŹ¾×°ÖƵē½ā£¬Ęä֊ijµē¼«ŌöÖŲa g£¬ĮķŅ»µē¼«ÉĻ²śÉś±ź×¼×“æöĻĀµÄĘųĢåV mL£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
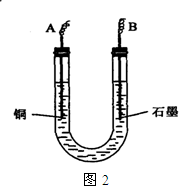
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖŌŚpHĪŖ4”«5µÄČÜŅŗÖŠ£¬Cu2+¼øŗõ²»Ė®½ā£¬¶ųFe3+¼øŗõĶźČ«Ė®½ā”£Ä³Ń§ÉśÄāÓƵē½āCuSO4ČÜŅŗµÄ·½·Ø²ā¶ØĶµÄĻą¶ŌŌ×ÓÖŹĮ攣øĆĶ¬Ń§ĻņpH=3.8Ėį»ÆµÄ”¢ŗ¬ÓŠFe2£ØSO4£©3ŌÓÖŹµÄCuSO4ČÜŅŗÖŠ¼ÓČė¹żĮæµÄŗŚÉ«·ŪÄ©X£¬³ä·Ö½Į°čŗó½«ĀĖŅŗÓĆĶ¼ĖłŹ¾×°ÖƵē½ā£¬Ęä֊ijµē¼«ŌöÖŲa g£¬ĮķŅ»µē¼«ÉĻ²śÉś±ź×¼×“æöĻĀµÄĘųĢåVmL”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A£®ŗŚÉ«·ŪÄ©XŹĒĢś·Ū
B£®Ķµē¼«Į¬½ÓµēŌ“Õż¼«
C£®ŹÆÄ«µē¼«ÉĻ·¢ÉśµÄ·“Ó¦ŹĒ4OH-£4e-=O2”ü£«2H2O
D£®ĶµÄĻą¶ŌŌ×ÓÖŹĮæµÄ¼ĘĖćŹ½ŹĒ![]()
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com